Figure 7. Schematic models of the integrin β1a-TMC in detergent micelles or in liposomes.
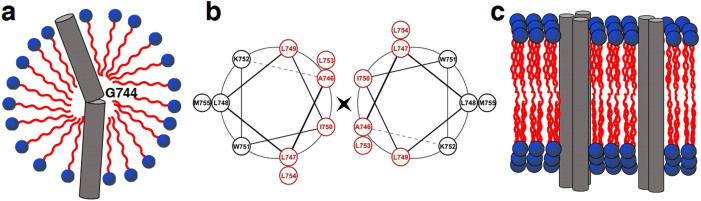
(a) The transmembrane domain of integrin β1a-TMC adopted a monomeric helix with a kink at residue G744. (b) Helical wheel diagram depicting the interface between the hydrophobic side chains of residues in the transmembrane domain (Ala746-Met755) of integrin β1a-TMC. Residues which might be involved in forming the hydrophobic interactions were colored in red. (c) Homo-oligomeric transmembrane domains of integrin β1a-TMC in liposomes. The clustering effect of transmembrane helices was represented by the formation of inter-helical dimers and trimers. The extracellular domain and cytoplasmic tail were eliminated for simplicity. Red areas represent the hydrophobic tail, and blue areas are the hydrophilic head for the illustration of detergent and phospholipid molecules.
