Abstract
Background:
Adrenergic receptor (ADR) genotypes are associated with heart failure (HF) and β-blocker response in adults. We assessed the influence of ADR genotypes in children with dilated cardiomyopathy (DCM).
Methods:
Ninety-one children with advanced DCM and 44 with stable DCM were genotyped for three ADR genotypes associated with HF risk in adults: α2cdel322-325, β1Arg389, and β2Arg16. Data were analyzed by genotype and β-blocker use. Mean age at enrollment was 8.5 y.
Results:
One-year event-free survival was 51% in advanced and 80% in stable DCM. High-risk genotypes were associated with higher left ventricular (LV) filling pressures, higher systemic and pulmonary vascular resistance, greater decline in LV ejection fraction (P < 0.05), and a higher frequency of mechanical circulatory support while awaiting transplant (P = 0.05). While β-blockers did not reduce HF severity in the overall cohort, in the subset with multiple high-risk genotypes, those receiving β-blockers showed better preservation of cardiac function and hemodynamics compared with those not receiving β-blockers (interaction P < 0.05).
Conclusion:
Our study identifies genetic risk markers that may help in the identification of patients at risk for developing decompensated HF and who may benefit from early institution of β-blocker therapy before progression to decompensated HF.
Dysregulation of the adrenergic system can contribute to the development and progression of heart failure (HF) through altered signaling of pre- and postsynaptic α- and β-adrenergic receptors (ADRs). Presynaptic α2C ADRs inhibit norepinephrine (NE) release, cardiac β1 ADRs are targets for NE-induced chronotropy and inotropy, and β2 ADRs mediate vascular smooth muscle relaxation (1,2,3,4). Activation of ADRs initially promotes maintenance of cardiac output but ultimately acts to accelerate HF progression (5). This forms the basis for the use of sympathetic antagonists such as β-blockers in the management of HF with reduced ejection fraction (HFREF) (6). Unlike in adults, β-blockers have not shown symptomatic or survival benefit in pediatric HFREF in the only prospective, placebo-controlled clinical trial to date (7). Lack of effective therapies contributes to poor outcomes with ~50% of children with dilated cardiomyopathy (DCM) either dying or requiring a heart transplant within 5 y of diagnosis (8).
Single-nucleotide polymorphisms (SNPs) in the ADR genes have been implicated in the progression of HFREF in adults. These polymorphisms include α2Cdel322-325 (associated with increased NE release) (5,9,10,11), β1Arg389 (associated with increased receptor sensitivity to NE) (12), and β2Arg16 (associated with receptor downregulation and impaired vasorelaxation) (13,14,15,16). Furthermore, homozygosity for both α2Cdel322-325 and β1Arg389 genotypes is associated with higher risk of HF in African Americans compared with Caucasians (17,18). Overall, the presence of genetic variations that increase cardiac sympathetic tone and/or peripheral vasoconstriction may increase the risk of HF progression by increasing cardiac work, myocardial oxygen consumption, and myocyte loss (4,19,20,21,22,23,24,25). The association of ADR polymorphisms with response to β-blockers is however conflicting (4,13,26,27,28,29). While the response to metoprolol and carvedilol, the most commonly used β-blockers in adult HFREF patients, was independent of ADR genotype, survival benefit with bucindolol was limited to the subset of patients carrying the β1Arg389 and α2Cdel322-325 genotype via downregulation of NE release (30,31,32).
Despite the role of these genetic variations in adult HFREF, their role in HF progression and response to β-blocker therapy in children is not known. Given widespread empiric use of β-blockers despite negative results of the pediatric carvedilol HF trial (7), knowledge of genetic markers that predict β-blocker response is particularly important since it would allow targeting of β-blockers only to potential responders and avoid futile therapy in nonresponders. However, this requires a pediatric-focused study since adult ADR genotype associations may not be reproducible in children since there are differences in ADR receptor subtype density, calcium transients, contractile force, and relaxation velocity (33). The objective of this study was to determine if variants in ADRA2c (α2Cdel322-325), ADRB1 (β1Arg389), and ADRB2 (β2Arg16) genes increase HF severity and influence β-blocker response in children with DCM.
Results
Allele Frequencies
The frequency of α2cdel322-325, β1Arg389, and β2Arg16 genotypes was similar in the 91 advanced HF patients (21, 48, and 48%, respectively) and 44 stable HF patients (22, 42, and 35%, respectively) and was comparable to previous studies in healthy adults (26,34,35). All genotypes were in Hardy–Weinberg equilibrium. Individual genotype assays did not yield results in five patients in the advanced HF cohort.
Clinical Characteristics
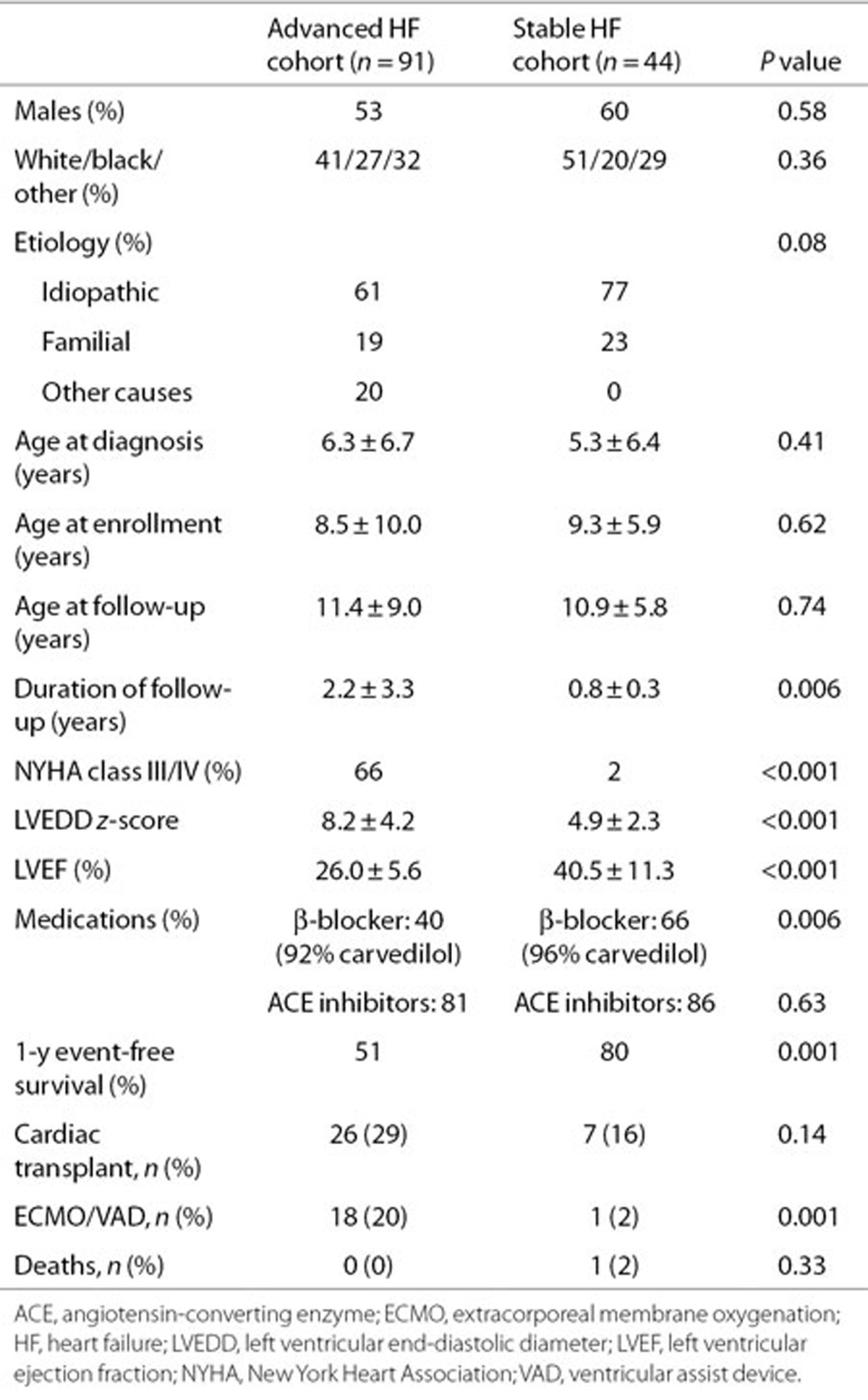
Clinical characteristics at enrollment are shown in Table 1. Demographic characteristics were similar in the two cohorts. There was no difference in age, gender, and etiology of HF at presentation among patients in the different genotype groups with 61% having sporadic idiopathic DCM, 19% familial, and 20% other causes including burnt out viral myocarditis (n = 8), neuromuscular disorder (n = 2), maternal lupus (n = 1), anthracycline cardiomyopathy (n = 3), and metabolic disease (n = 4). In the advanced HF group, the LV end-diastolic volume z score was 5.2 ± 2.6, and the shortening fraction was 13.1 ± 5.8%. Detailed echocardiographic data were available in the stable HF group as part of the ventricular volume variability (VVV) study (Table 2). Baseline clinical and echocardiographic characteristics of patients in this genetic substudy were comparable to the parent VVV patient cohort from which this subset was derived (data not shown).
Table 1. Clinical characteristics at enrollment.
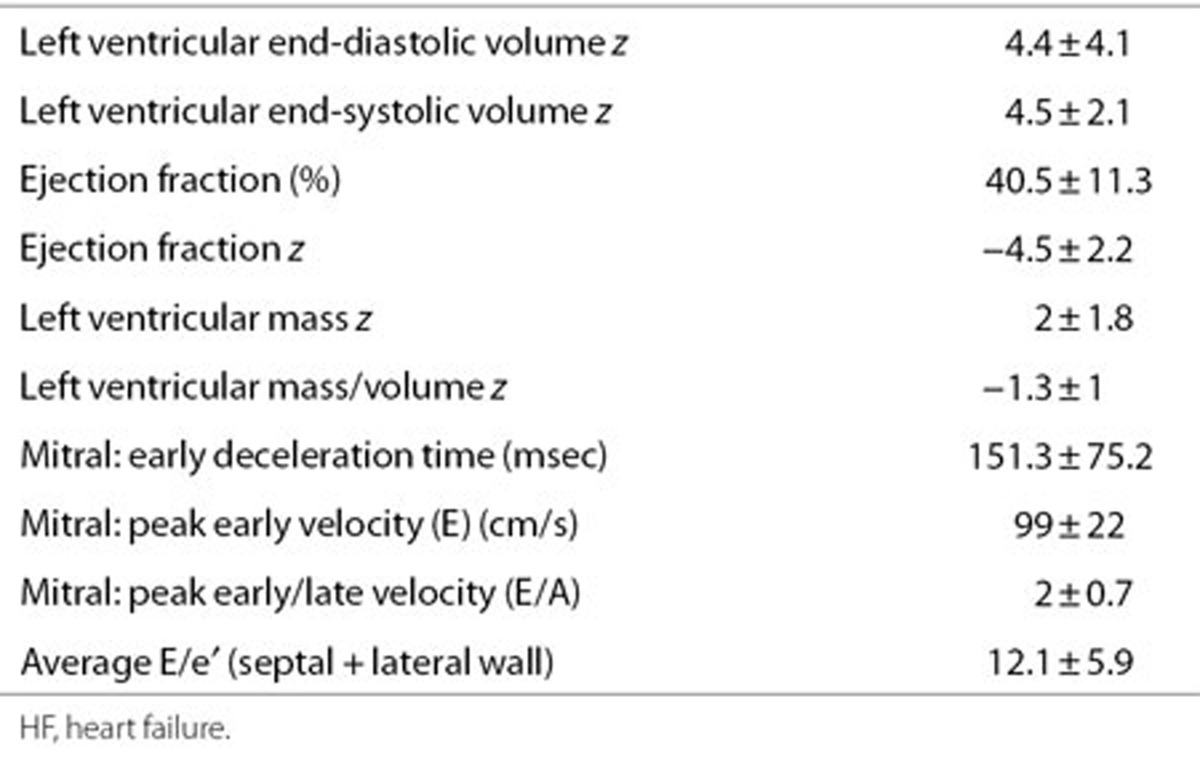
Table 2. Echocardiographic characteristics at enrollment in the stable HF cohort (n = 44).
Genotype Association With Hemodynamics, Outcomes, and Response to β-Blocker Therapy (Advanced HF)
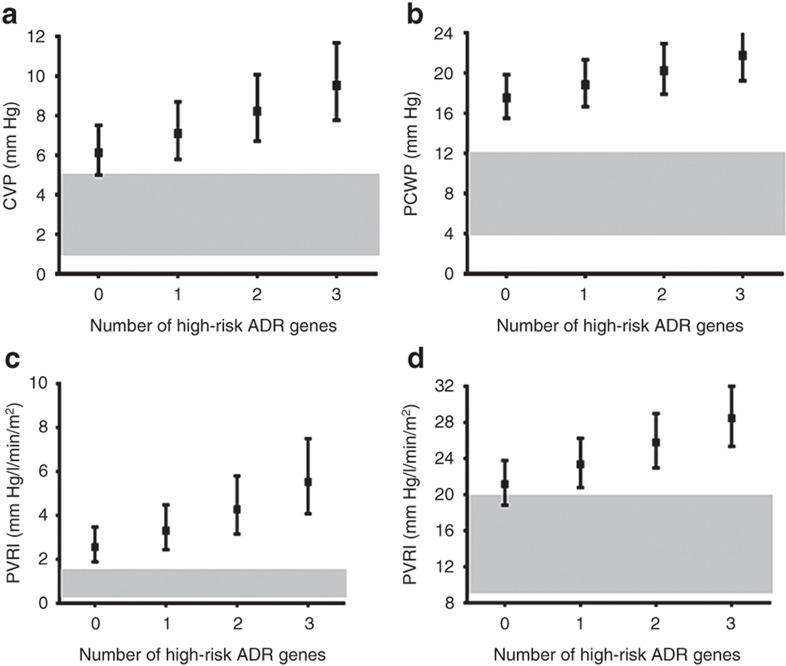
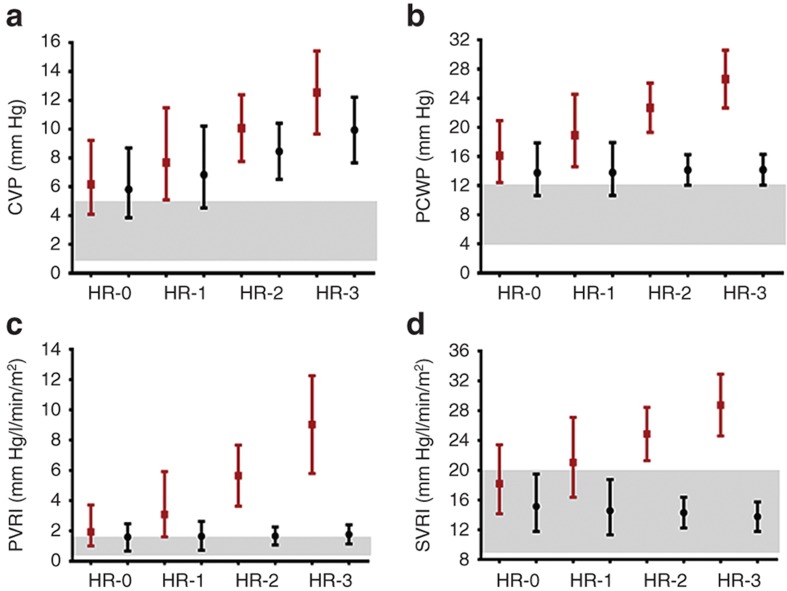
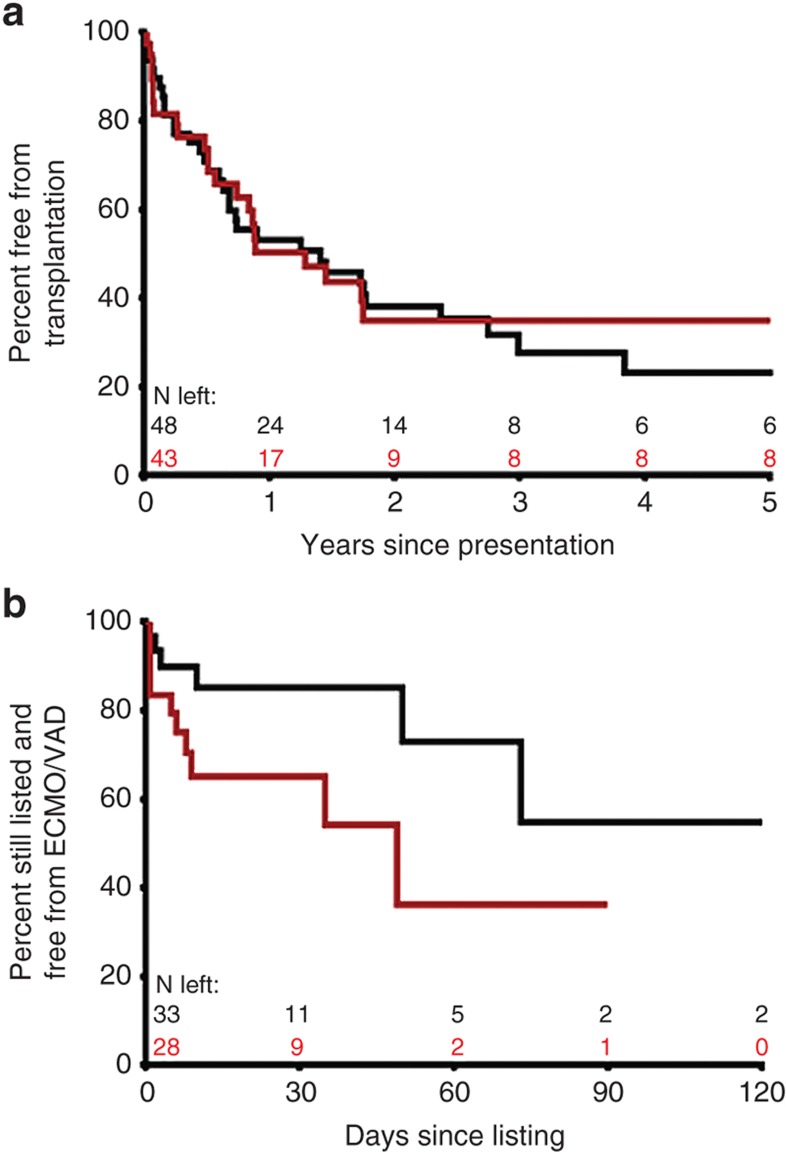
During follow-up, 57 of the 91 patients in the advanced HF cohort underwent cardiac catheterization to assess HF severity. Median time from diagnosis to cardiac catheterization was 3 mo reflecting a cohort presenting in advanced HF. Average hemodynamic measurements at cardiac catheterization were central venous pressure, 7 ± 4 mm Hg; pulmonary capillary wedge pressure, 19 ± 8 mm Hg; indexed pulmonary vascular resistance, 3.7 ± 2.5 mm Hg/l/min/m2, indexed systemic vascular resistance, 24 ± 10 mm Hg/l/min/m2, and cardiac index, 3.1 ± 1.5 l/min/m2. There was a significant association between ADR genotypes and hemodynamic severity of HF manifested by higher pulmonary capillary wedge pressure, indexed pulmonary vascular resistance, and indexed systemic vascular resistance in patients with a higher number of ADR high-risk genotypes. There was an additive effect of multiple high-risk genotypes with a linear increase in central venous pressure, pulmonary capillary wedge pressure, indexed pulmonary vascular resistance, and indexed systemic vascular resistance with increasing number of high-risk genotypes (Figure 1). Since factors like age, use of general anesthesia, or oxygen supplementation during catheterization can independently influence cardiac hemodynamics, we analyzed but found no influence of these factors on the association between ADR genotype and hemodynamic severity of HF. Forty percent of patients were receiving β-blockers at enrollment (92% carvedilol; mean dose: 0.57 ± 0.53 mg/kg/d). The subset of patients with the high-risk genotype receiving β-blockers showed an improvement in their hemodynamics compared with those not receiving β-blockers (genotype group × β-blocker use, interaction P values ≤ 0.05; Figure 2). There was no significant difference in freedom from transplantation between the two genotype groups (hazard ratio: 0.88; 95% confidence interval: 0.52–1.46; Figure 3a). There was, however, a lower freedom from ventricular assist device/extracorporeal membrane oxygenation in listed patients with ≥2 ADR high-risk genotypes awaiting transplantation (hazard ratio: 2.57; 95% confidence interval: 1.05–7.23; Figure 3b).
Figure 1.
Cumulative effect of multiple adrenergic risk genotypes on cardiac hemodynamics (advanced HF cohort). The squares represent the point estimates from regression models, and the solid bars represent the 95% confidence interval around that estimate (n = 57). A higher number of adrenergic high-risk genotypes were associated with (a) higher CVP (P = 0.02), (b) higher PCWP (P = 0.06), (c) higher PVRI (P = 0.004), and (d) higher SVRI (P = 0.005). The gray zones represent normal hemodynamic ranges, i.e., CVP, 1–5 mm Hg; PCWP, 4–12 mm Hg; PVRI, 0.25–1.6 mm Hg/l/min/m2; and SVRI, 9–20 mm Hg/l/min/m2. ADR, adrenergic receptor; CVP, central venous pressure; PCWP, pulmonary capillary wedge pressure; PVRI, indexed pulmonary vascular resistance; SVRI, indexed systemic vascular resistance.
Figure 2.
Interaction between adrenergic genotype and hemodynamic response to β-blockers (advanced HF cohort). The markers represent the point estimates (red: not on β-blockers, n = 27; black: on β-blockers, n = 28) from regression models, and the solid bars represent the 95% confidence interval around that estimate. Patients not receiving β-blockers showed (a) higher CVP (P = 0.02), (b) higher PCWP (P = 0.004), (c) higher PVRI (P = 0.0003), and (d) higher SVRI (P = 0.009) for every additional high-risk genotype. This increase in filling pressures and resistances was either blunted (CVP) or absent (PCWP, PVRI, and SVRI) in patients with high-risk genotypes receiving β-blockers. There was a significant interaction between ADR genotype and β-blocker therapy for PCWP (interaction P = 0.05), PVRI (interaction P = 0.03), and SVRI (interaction P = 0.02). Red, no β-blocker, black; β-blocker. The gray zones represent normal hemodynamic ranges, i.e., CVP, 1–5 mm Hg; PCWP, 4–12 mm Hg; PVRI, 0.25–1.6 mm Hg/l/min/m2; and SVRI, 9–20 mm Hg/l/min/m2. ADR, adrenergic receptor; CVP, central venous pressure; HR, high-risk genotype numbers; PCWP, pulmonary capillary wedge pressure; PVRI, indexed pulmonary vascular resistance; SVRI, indexed systemic vascular resistance.
Figure 3.
Adrenergic genotype and event-free survival (advanced HF cohort). (a) Time to transplantation during follow-up was not different between patients with <2 (black, n = 48) vs. ≥2 (red, n = 43) ADR high-risk genotypes (hazard ratio: 0.88; 95% confidence interval: 0.52–1.46). (b) Patients listed for transplantation with ≥2 ADR high-risk genotypes (n = 28) showed lower freedom from mechanical circulatory support (VAD/ECMO) as a bridge to transplantation compared with those with <2 risk genotypes (n = 33) despite similar waiting times (hazard ratio: 2.57; 95% confidence interval: 1.05–7.23). Number of patients remaining at each time point are shown in black for patients with <2 high-risk genotypes and in red for patients with ≥2 high-risk genotypes. ADR, adrenergic receptor; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device.
Genotype Association With Change in LV Systolic and Diastolic Function Stratified by β-Blocker Therapy (Stable HFREF)
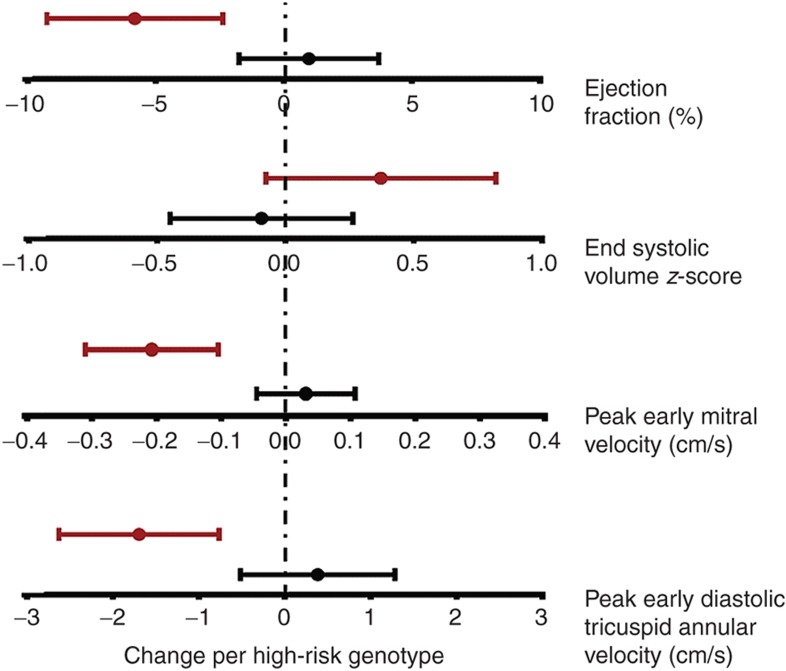
To assess the influence of ADR genotypes in earlier stages of HF, we enrolled 44 patients with stable HF. Sixty-six percent were receiving β-blockers at enrollment (96% carvedilol; mean dose: 0.44 ± 0.28 mg/kg/d). There was no association between ADR genotypes and New York Heart Association/Ross HF severity score. Regression analyses were performed to assess association of ADR genotypes with change in ventricular systolic and diastolic function on serial echocardiograms in 35 stable HF patients in whom serial echocardiographic data were available. Figure 4 shows the change in echocardiographic measurements during follow-up for each additional ADR risk genotype stratified by β-blocker use. There was a greater decline in LV ejection fraction for every additional ADR risk genotype, but this association was not seen in patients receiving β-blockers (genotype group × β-blocker use, interaction P = 0.003 vs. those not receiving β-blockers). There was a greater increase in LV end-systolic volume z-score for every additional ADR risk genotype, and this association was seen independent of β-blocker use (interaction P = 0.12). There was a greater decline in peak mitral E wave velocity and in early diastolic tricuspid annular velocity for every additional ADR risk genotype, but this association was not seen in patients receiving β-blockers (interaction P < 0.001 vs. those not receiving β-blockers). Interaction of individual ADR genotypes with β-blockers was not assessed due to the small sample size. Overall, these results suggest that patients with a higher number of ADR high-risk genotypes show more rapid progression of systolic and diastolic dysfunction but that this progression may be prevented by β-blocker therapy with an improvement or stabilization in LV size and function.
Figure 4.
Adrenergic genotype and change in ventricular systolic and diastolic dysfunction (stable HF cohort). Regression analyses were performed to assess association of ADR genotypes with change in ventricular systolic and diastolic function on serial echocardiograms in 35 stable HF patients. Graphs show parameter estimate of change in echocardiographic measurements for each additional ADR risk genotype stratified by β-blocker use. Red, on no β-blockers; black, on β-blockers. There was a greater decline in LV ejection fraction for every additional ADR risk genotype, but this association was not seen in patients receiving β-blockers (interaction P = 0.003 vs. those not receiving β-blockers). There was a greater increase in LV end-systolic volume z-score for every additional ADR risk genotype, but this did not reach statistical significance between those receiving vs. those not receiving β-blockers (interaction P = 0.12). There was a greater decline in peak mitral E wave velocity for every additional ADR risk genotype, but this association was not seen in patients receiving β-blockers (interaction P < 0.001 vs. those not receiving β-blockers). There was a greater decline in early diastolic tricuspid annular velocity with each additional ADR risk genotype, but this association was not seen in patients receiving β-blockers (interaction P = 0.001 vs. those not receiving β-blockers) (n = 7 for 0, n = 22 for 1, n = 22 for 2, and n = 4 for 3 risk genotypes).
Discussion
DCM is the most common form of cardiomyopathy and the leading indication for transplantation in adults and children. Almost 50% of children with DCM either die or require a heart transplant within 5 y of diagnosis (3,8). Carvedilol is widely used but has failed to show clinical benefit in one of the largest clinical trials of pediatric HF to date, although the trial did not evaluate response by genotype (7). Our study identifies genetic risk markers that may help in the identification of patients at risk for developing decompensated HF who may benefit from early institution of β-blocker therapy before progression to decompensated HF.
An important finding of our study is the association of high-risk ADR genotypes with hemodynamic severity of HF in patients with advanced DCM. Those with a higher number of ADR high-risk genotypes had higher left- and right-sided filling pressures and higher systemic and pulmonary vascular resistance, faster progression of LV systolic and diastolic dysfunction, as well as a higher incidence of acute decompensation requiring mechanical circulatory support during follow-up. The combined physiologic effects of these genotypes are most likely related to an increase in the release and responsiveness to NE as well as a decrease in the sensitivity of β-ADRs which can contribute to impaired myocardial contractility, impaired peripheral vasodilation, and progression of cardiac dysfunction (5,18). Our results suggest that genotyping for ADR polymorphisms may help in the early identification of patients at risk for decompensated HF who may benefit from closer monitoring and timely listing for transplantation before hemodynamic decompensation.
We further evaluated the pharmacogenetic interactions between the ADR genotypes and response to β-blocker therapy. Although our numbers are small, we found results similar to the carvedilol trial—patients receiving β-blockers did not show improvement in HF severity in the overall cohort. However, when stratified by ADR risk genotypes, β-blocker therapy was associated with better hemodynamic adaptation in the high-risk genotype groups. This suggests that the benefit of β-blocker therapy in pediatric DCM may be seen primarily in those in whom disease progression is mediated by dysregulated ADR signaling likely due to variant ADR genotypes. This is consistent with the findings by Liggett et al. (31) who showed that the β-blocker, bucindolol, a sympatholytic agent, was effective in improving HF survival only in adults with the β1Arg389 genotype, not in those with the β1Gly389 genotype.
In light of the observable benefit of β-blocker therapy in advanced HF patients with high-risk genotypes, we analyzed if β-blocker therapy would show a similar benefit in patients with earlier stages of DCM. We found that patients with high-risk genotypes showed a decline in ejection fraction and diastolic function during follow-up but that this decline was attenuated, and even improved, in high-risk genotype patients receiving β-blockers. These findings suggest that β-blocker therapy in the subset of stable HF patients with high-risk genotypes may not only prevent decline in function but may also lead to recovery of ventricular function and reverse remodeling. This exciting finding highlights the importance of early institution of β-blocker therapy in this high-risk subset to prevent disease progression. This finding is particularly important in light of the results of the only randomized trial with β-blockers in children with HF that failed to reproduce the benefits seen in adult HF (7). Our findings suggest that while not all pediatric DCM patients benefit from β-blocker therapy, the subgroup of patients with high-risk ADR genotypes may show benefit. Although genotype did not influence carvedilol response in adult studies, children may be more catecholamine dependent compared to adults, and therefore, the impact of receptor genotype may be greater than that in adults. Given the considerable phenotypic heterogeneity in sporadic as well as inherited DCM where considerable intrafamilial variability exists, knowledge of disease modifiers may enable better risk prediction not only in sporadic but also in familial cases and, more importantly, facilitate risk modification strategies by targeting therapies to potential responders.
Limitations
Since patients were not always enrolled at the time of initial diagnosis of DCM, this may introduce a survivor bias in our cohort. The study was not powered to analyze the effect of underlying genetic mutation type on HF severity and progression. The study was also limited by the relatively short mean follow-up of 0.8 y in the stable HF cohort; therefore, longer-term outcomes were not assessed. Although results from our study show a difference in rates of acute decompensation requiring mechanical circulatory support between the two genotype risk groups, these results require further validation in a larger patient cohort.
Conclusions
The characterization of the ADR genotype may aid in risk stratifying patients with DCM who are more likely to progress to decompensated HF. The preferential responsiveness of patients with high-risk genotypes to β-blocker therapy highlights the importance of early institution of β-blocker therapy in genetically susceptible patients while avoiding futile therapy in those unlikely to respond. Prospective, randomized, genotype-guided trials of β-blocker therapy in this high-risk subset are needed to validate these observations and develop pharmacogenetic-guided therapies in this vulnerable cohort.
Methods
Study Population
The study included patients with advanced DCM from a single US institution and patients with stable DCM from a multi-institutional North American cohort. The advanced DCM group consisted of 91 DCM patients aged <21 y, who were on medical therapy with symptomatic HF and undergoing evaluation for transplantation at the Columbia University Medical Center (2002–2005). The stable DCM group included 44 DCM patients with stable HFREF enrolled in a multicenter Pediatric Heart Network observational study of DCM, the VVV study (accrual 2005–2007; Clinical Trials Registration: #NCT00123071). Detailed design of the Pediatric Heart Network study has been previously published (36). The inclusion criteria for the VVV study from which this stable HF cohort was derived were pediatric DCM patients, disease duration >2 mo, on outpatient medical therapy, no hemodynamic instability, and not being assessed or listed for transplant (due to the intent to assess longitudinal natural history). Patients were enrolled across eight North American centers and followed with serial echocardiography for 18 mo. Four of eight VVV sites participated in a genetic substudy and provided DNA samples from enrolled patients—Columbia University Medical Center, Children's Hospital Boston, Children's Hospital of Philadelphia, and Hospital for Sick Children, Toronto. In both cohorts, children with active myocarditis, hypertrophic or restrictive cardiomyopathy, or congenital heart disease were excluded. Informed consent was obtained from the parents and assent from older subjects. The study was approved by the local Institutional Review Boards of all participating sites—Columbia University Medical Center, Children's Hospital Boston, Children's Hospital of Philadelphia, and Hospital for Sick Children, Toronto.
Clinical Data
Age at presentation, gender, race, ethnicity, New York Heart Association or Ross HF class at presentation, medications, echocardiographic variables at enrollment, hemodynamic data on cardiac catheterization (advanced HF cohort only), frequency of ventricular assist device or extracorporeal membrane oxygenation implantation, and cardiac transplantation or death during study period were analyzed in high- and low-risk genotype groups. Echocardiograms in the advanced HF group were performed using a standardized clinical protocol. Hemodynamic data included central venous pressure, pulmonary capillary wedge pressure, indexed pulmonary vascular resistance, indexed systemic vascular resistance, and cardiac index. For patients in the stable HF cohort, centralized clinical data collection was done by the New England Research Institute (Watertown, MA), and all echocardiograms were acquired using a standardized protocol with central analysis by a single reviewer at the echocardiographic core laboratory at Children's Hospital Boston. Only patients with at least two echocardiograms, a minimum of 3 mo apart, were included in the final echocardiographic analysis. The following variables were assessed: LV end-diastolic diameter and volume z-score, LV end-systolic diameter and volume z-score, LV shortening fraction, LV ejection fraction, and peak early and late diastolic filling velocities. While inflow velocities are not reliable markers for clinical deterioration, they provide incremental predictive power for cardiac mortality compared with clinical data and LV ejection fraction alone in adult studies with only limited tissue Doppler data available in children with DCM (37). The interpreters of the echocardiograms were blinded to the genotype data (36).
Candidate Genes and SNP Selection
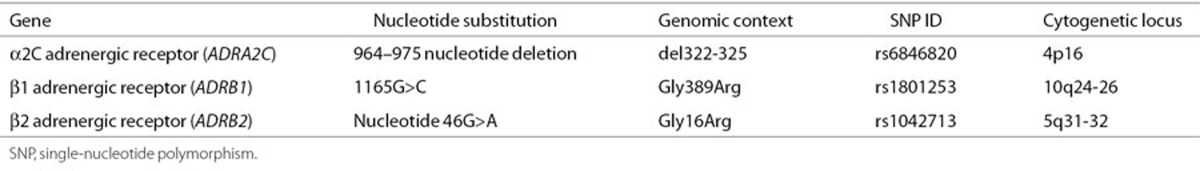
Three candidate ADR genes (ADRA2c, ADRB1, and ADRB2) were selected based on their role in modulating sympathetic tone and association with adult HF (5,9,12,14,16). Genotypes previously associated with HF in adults were defined as high-risk genotypes for the purpose of this study (α2Cdel322-325-ADRA2c, β1Arg389-ADRB1, and β2Arg16-ADRB2; Table 3) and evaluated for association with echocardiographic evidence of disease progression, hemodynamic parameters, and clinical outcomes (4,5,15,34,38).
Table 3. Candidate genes and polymorphisms.
Genotyping
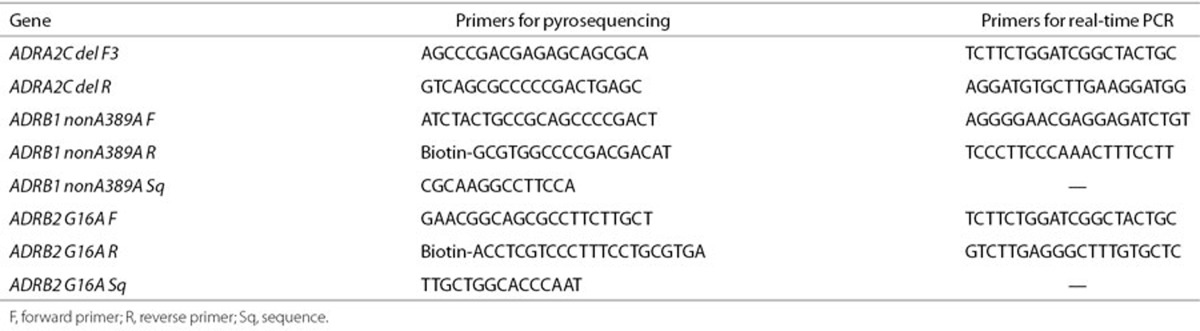
Genomic DNA was isolated from whole blood (Gentra Systems, Minneapolis, MN). ADRA2c SNP was genotyped by end-labeling primers (Table 4) with γ-32P-ATP (PerkinElmer Life Science, Boston, MA) using T4 polynucleotide kinase (Promega, Madison, WI) and using PCR to amplify the genomic fragment. The PCR products were resolved on an 8% nondenaturing polyacrylamide gel to resolve the size difference. ADRB1 and ADRB2 SNPs were genotyped individually using pyrosequencing (PSQ96; Biotage, Westborough, MA) with a vacuum system with streptavidin sepharose beads (Amersham Biosciences AB, Uppsala, Sweden). PCR reactions consisted of 5 pmol of each of the appropriate forward and reverse primers (Table 4), 1 U AccuPrime GC-Rich DNA polymerase (Invitrogen, Carlsbad, CA), 1× buffer A, and 30 ng of genomic DNA in a 25 ml reaction volume for 30 cycles at an annealing temperature of 62 °C for ADRA2c and ADRB2 and touch-down from 65 to 50 °C for ADRB1. Each assay was performed in duplicate to ensure accuracy.
Table 4. Primers used in pyrosequencing and real-time PCR.
Statistical Analysis
Hardy–Weinberg equilibrium was determined using Pearson χ2 analysis of actual and predicted genotypes. Presence of a single risk allele was defined as a high-risk genotype. Data are reported as means with SDs, median with minimum and maximum and frequencies as appropriate. The combined effect of multiple high-risk genotypes on echocardiographic and hemodynamic variables was assessed in a univariate regression model using maximum likelihood estimates and appropriate mathematical transformations to normalize the distribution of the response variable. Regression models adjusted for repeated measures through a compound symmetry covariance structure were used to determine the effect of ADR genotypes on serial echocardiographic measurements and on hemodynamic measures. The associations between high-risk genotype and outcomes were also tested in multivariable models including an interaction variable between genotype and β-blocker use at enrollment. Age and time since diagnosis were also included in the model. HF progression was defined as worsening hemodynamics and/or echocardiographic progression of LV dilation and dysfunction. β-Blocker responsiveness was defined as an improvement or stabilization in LV size and function. Event-free survival, i.e., freedom from heart transplantation and freedom from extracorporeal membrane oxygenation/ventricular assist device while listed for transplantation was modeled using Kaplan–Meier nonparametric estimates, and distributions of time to event were compared using the log-rank test. Follow-up time was censored at death or transplant when analyzing time to extracorporeal membrane oxygenation/ventricular assist device initiation. All statistical analyses were performed using SAS Statistical Software v9.1 (The SAS Institute, Cary, NC).
Statement of Financial Support
This work was supported by the US National Institutes of Health (NIH; Bethesda, MD, USA; grant number: RR00645); Children's Cardiomyopathy Foundation, Tenafly, NJ, USA; and the Syde Hurdus Foundation, New York, NY, USA. This work was also supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068279, HL068285, HL068290, and HL068288), NIH.
Disclosure
None.
Acknowledgments
The authors thank Claudia Fontes, BA, and Alisa Nakamine, BA, for help with sample processing for genotyping; Patricia Lanzano and Liyong Deng for genotyping of the primary cohort; Tara Paton for genotyping the secondary cohort; and Shing Lee for help with statistical analysis. The authors also thank the SickKids Labatt Family Heart Centre Biobank Registry for access to patient DNA. This work was presented, in part, at the Annual Scientific Sessions of the American Heart Association, November 2011.
References
- Majewski H. Modulation of noradrenaline release through activation of presynaptic beta-adrenoreceptors. J Auton Pharmacol. 1983;3:47–60. doi: 10.1111/j.1474-8673.1983.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Brede M, Wiesmann F, Jahns R.et al.Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure Circulation 20021062491–6. [DOI] [PubMed] [Google Scholar]
- Hein L, Kobilka BK. Adrenergic receptor signal transduction and regulation. Neuropharmacology. 1995;34:357–66. doi: 10.1016/0028-3908(95)00018-2. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Smirk B, Finch S, Williams C, Esler MD. Interaction between cardiac sympathetic drive and heart rate in heart failure: modulation by adrenergic receptor genotype. J Am Coll Cardiol. 2004;44:2008–15. doi: 10.1016/j.jacc.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–42. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN.et al.The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group N Engl J Med 19963341349–55. [DOI] [PubMed] [Google Scholar]
- Shaddy RE, Boucek MM, Hsu DT.et al.; Pediatric Carvedilol Study Group Carvedilol for children and adolescents with heart failure: a randomized controlled trial JAMA 20072981171–9. [DOI] [PubMed] [Google Scholar]
- Daubeney PE, Nugent AW, Chondros P.et al.; National Australian Childhood Cardiomyopathy Study Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study Circulation 20061142671–8. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–4. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000;275:23059–64. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- Minatoguchi S, Ito H, Ishimura K.et al.Modulation of noradrenaline release through presynaptic alpha 2-adrenoceptors in congestive heart failure Am Heart J 19951303 Pt 1516–21. [DOI] [PubMed] [Google Scholar]
- Sandilands AJ, O'Shaughnessy KM, Brown MJ. Greater inotropic and cyclic AMP responses evoked by noradrenaline through Arg389 beta 1-adrenoceptors versus Gly389 beta 1-adrenoceptors in isolated human atrial myocardium. Br J Pharmacol. 2003;138:386–92. doi: 10.1038/sj.bjp.0705030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groote P, Lamblin N, Helbecque N.et al.The impact of beta-adrenoreceptor gene polymorphisms on survival in patients with congestive heart failure Eur J Heart Fail 20057966–73. [DOI] [PubMed] [Google Scholar]
- La Rosée K, Huntgeburth M, Rosenkranz S, Böhm M, Schnabel P. The Arg389Gly beta1-adrenoceptor gene polymorphism determines contractile response to catecholamines. Pharmacogenetics. 2004;14:711–6. doi: 10.1097/00008571-200411000-00001. [DOI] [PubMed] [Google Scholar]
- Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- Dishy V, Sofowora GG, Xie HG.et al.The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization N Engl J Med 20013451030–5. [DOI] [PubMed] [Google Scholar]
- Liggett SB. Polymorphisms of the beta2-adrenergic receptor. N Engl J Med. 2002;346:536–8. doi: 10.1056/NEJM200202143460718. [DOI] [PubMed] [Google Scholar]
- Metra M, Zani C, Covolo L.et al.Role of beta1- and alpha2c-adrenergic receptor polymorphisms and their combination in heart failure: a case-control study Eur J Heart Fail 20068131–5. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–63. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- Unverferth DV, Magorien RD, Moeschberger ML, Baker PB, Fetters JK, Leier CV. Factors influencing the one-year mortality of dilated cardiomyopathy. Am J Cardiol. 1984;54:147–52. doi: 10.1016/0002-9149(84)90320-5. [DOI] [PubMed] [Google Scholar]
- Campana C, Gavazzi A, Berzuini C.et al.Predictors of prognosis in patients awaiting heart transplantation J Heart Lung Transplant 199312756–65. [PubMed] [Google Scholar]
- Morley D, Brozena SC. Assessing risk by hemodynamic profile in patients awaiting cardiac transplantation. Am J Cardiol. 1994;73:379–83. doi: 10.1016/0002-9149(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Stevenson LW, Tillisch JH, Hamilton M.et al.Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy Am J Cardiol 1990661348–54. [DOI] [PubMed] [Google Scholar]
- Nohria A, Tsang SW, Fang JC.et al.Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure J Am Coll Cardiol 2003411797–804. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT.et al.Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure N Engl J Med 1984311819–23. [DOI] [PubMed] [Google Scholar]
- Liggett SB. beta(2)-adrenergic receptor pharmacogenetics. Am J Respir Crit Care Med. 2000;161 3 Pt 2:S197–201. doi: 10.1164/ajrccm.161.supplement_2.a1q4-10. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Terra SG. Beta-adrenergic receptor polymorphisms: cardiovascular disease associations and pharmacogenetics. Pharm Res. 2002;19:1779–87. doi: 10.1023/a:1021477021102. [DOI] [PubMed] [Google Scholar]
- Mialet Perez J, Rathz DA, Petrashevskaya NN.et al.Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure Nat Med 200391300–5. [DOI] [PubMed] [Google Scholar]
- Lobmeyer MT, Gong Y, Terra SG.et al.Synergistic polymorphisms of beta1 and alpha2C-adrenergic receptors and the influence on left ventricular ejection fraction response to beta-blocker therapy in heart failure Pharmacogenet Genomics 200717277–82. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Daniels SE, Elashoff M.et al.Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol J Am Coll Cardiol 200852644–51. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Mialet-Perez J, Thaneemit-Chen S.et al.A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure Proc Natl Acad Sci USA 200610311288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow MR, Murphy GA, Krause-Steinrauf H.et al.An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure Circ Heart Fail 2010321–8. [DOI] [PubMed] [Google Scholar]
- Molenaar P, Bartel S, Cochrane A.et al.Both beta(2)- and beta(1)-adrenergic receptors mediate hastened relaxation and phosphorylation of phospholamban and troponin I in ventricular myocardium of Fallot infants, consistent with selective coupling of beta(2)-adrenergic receptors to G(s)-protein Circulation 20001021814–21. [DOI] [PubMed] [Google Scholar]
- Leineweber K, Büscher R, Bruck H, Brodde OE. Beta-adrenoceptor polymorphisms. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:1–22. doi: 10.1007/s00210-003-0824-2. [DOI] [PubMed] [Google Scholar]
- Moore JD, Mason DA, Green SA, Hsu J, Liggett SB. Racial differences in the frequencies of cardiac beta(1)-adrenergic receptor polymorphisms: analysis of c145A>G and c1165G>C. Hum Mutat. 1999;14:271. doi: 10.1002/(SICI)1098-1004(1999)14:3<271::AID-HUMU14>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Colan SD, Shirali G, Margossian R.et al.The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy J Am Soc Echocardiogr 201225842–54.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yip GW, Wang AY.et al.Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value J Am Coll Cardiol 200341820–6. [DOI] [PubMed] [Google Scholar]
- Grocott HP, White WD, Morris RW.et al.; Perioperative Genetics and Safety Outcomes Study (PEGASUS) Investigative Team Genetic polymorphisms and the risk of stroke after cardiac surgery Stroke 2005361854–8. [DOI] [PubMed] [Google Scholar]