Abstract
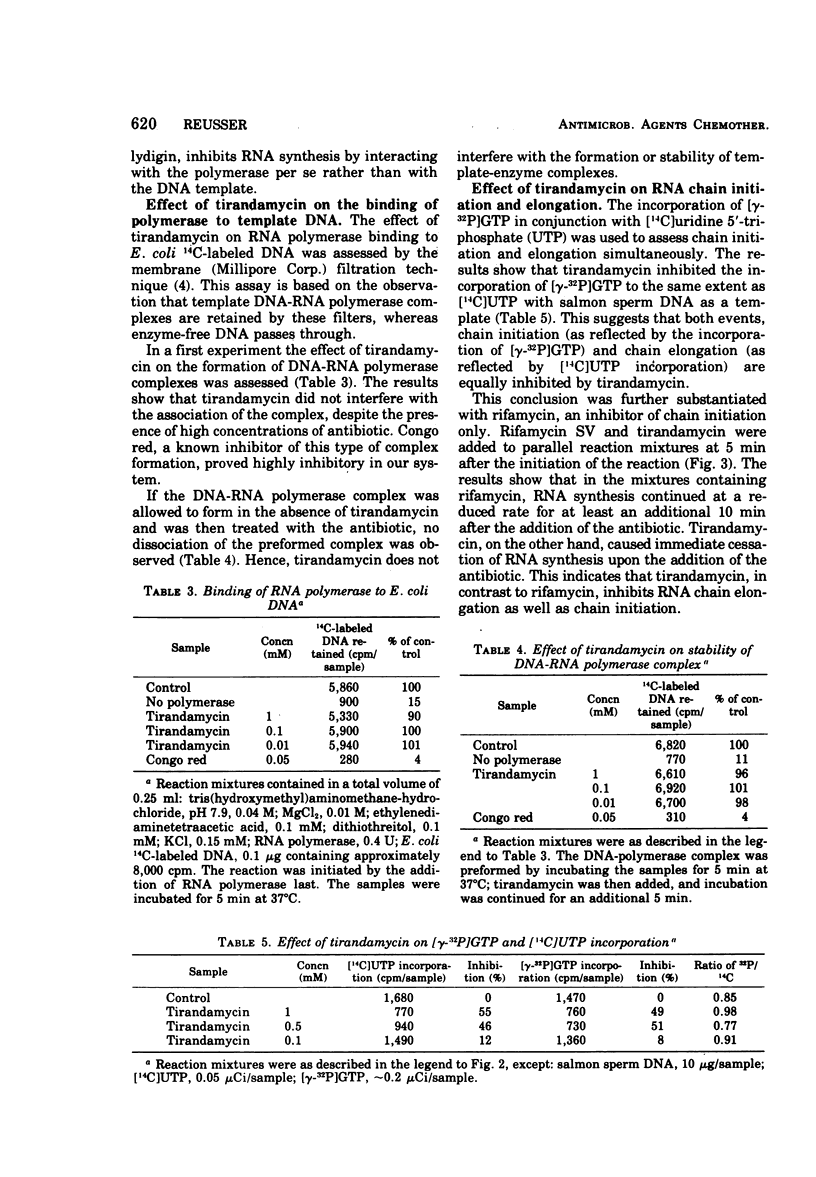
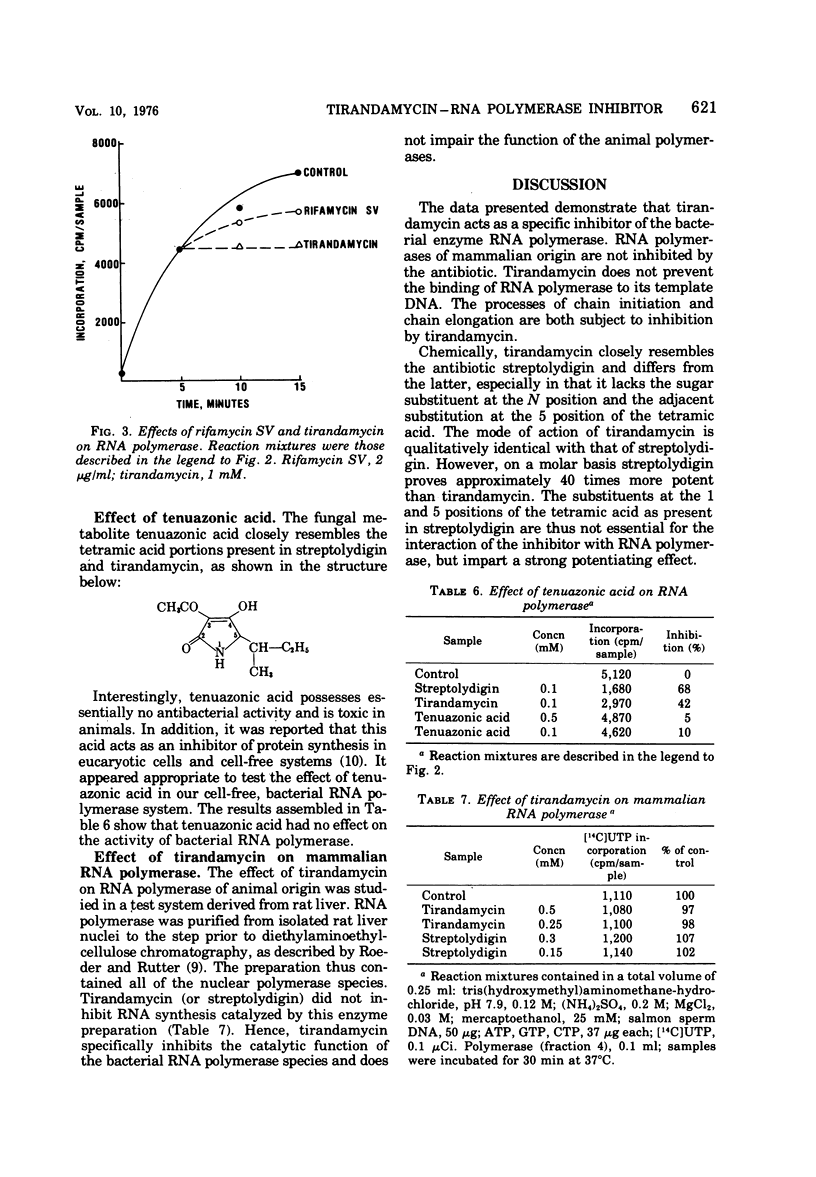
The antibiotic tirandamycin (a 3-acyltetramic acid structurally related to streptolydigin) specifically inhibits transcription by interfering with the function of bacterial ribonucleic acid polymerase. Ribonucleic acid polymerases from rat liver nuclei are not subject to tirandamycin inhibition. Qualitatively, the mode of action of the antibiotic is identical to that of streptolydigin in inhibiting chain initiation as well as chain elongation during the transcriptional process. However, tirandamycin is approximately 40 times less potent than streptolydigin. The structures of the 3-acyl groups of the two acyltetramic acid antibiotics tirandamycin and streptolydigin differ only slightly in the degree of oxidation of the terminal dioxabicyclo (3.1)nonane system and possess the same stereochemistry (D. J. Duchamp, A. R. Branfman, A. C. Button, and K. L. Rinehart, 1973). More significantly, major differences occur at the 1 and 5 positions of the tetramic acids. Tirandamycin contains no substituents; streptolydigin contains a substituted acetamide function at position 5 and a sugar moiety at position 1. The lack of substituents at the 1 and 5 positions of the tetramic acid portion in tirandamycin is probably responsible for the reduced biopotency of tirandamycin as compared with streptolydigin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassani G., Burgess R. R., Goodman H. M., Gold L. Inhibition of RNA polymerase by streptolydigin. Nat New Biol. 1971 Apr 14;230(15):197–200. doi: 10.1038/newbio230197a0. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Green M. H. Early-late transcription switch: isolation of a lambda DNA-RNA polymerase complex active in the synthesis of late RNA. Biochem Biophys Res Commun. 1968 Jun 28;31(6):919–924. doi: 10.1016/0006-291x(68)90540-8. [DOI] [PubMed] [Google Scholar]
- Duchamp D. J., Branfman A. R., Button A. C., Rinehart K. L., Jr X-ray structure of tirandamycic acid p-bromophenacyl ester. Complete stereochemical assignments of tirandamycin and streptolydigin. J Am Chem Soc. 1973 Jun 13;95(12):4077–4078. doi: 10.1021/ja00793a058. [DOI] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- MacKellar F. A., Grostic M. F., Olson E. C., Wnuk R. J. Tirandamycin. I. Structure assignment. J Am Chem Soc. 1971 Sep;93(19):4943–4945. doi: 10.1021/ja00748a067. [DOI] [PubMed] [Google Scholar]
- Meyer C. E. Tirandamycin, a new antibiotic isolation and characterization. J Antibiot (Tokyo) 1971 Aug;24(8):558–560. doi: 10.7164/antibiotics.24.558. [DOI] [PubMed] [Google Scholar]
- Reusser F. Tirandamycin: inhibition of ribonucleic Acid polymerase. Infect Immun. 1970 Jul;2(1):77–81. doi: 10.1128/iai.2.1.77-81.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]


