Figure 4.
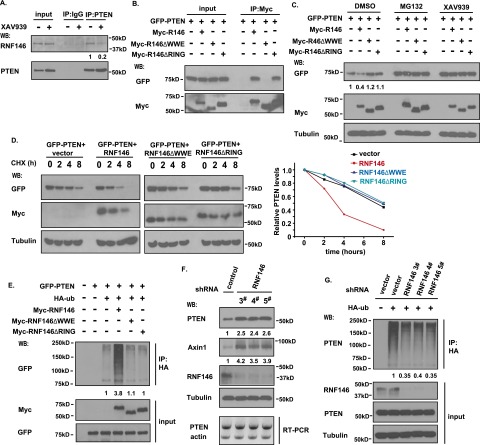
The E3 ligase RNF146 is involved in tankyrase-mediated PTEN ubiquitination and degradation. (A) Interaction between PTEN and RNF146. HCT116 cells were treated without or with 5 μM XAV939 for 6 h. Cell lysates were immunoprecipitated with anti-PTEN or control antibody followed by Western blotting using the indicated antibodies. (B) The WWE domain of RNF146 is required for its binding to PTEN. 293T cell were transfected with constructs encoding GFP-tagged PTEN together with vector alone or constructs encoding myc-tagged wild-type RNF146, the RNF146-△WWE mutant, or the RNF146-△RING mutant. Immunoprecipitation reactions were conducted using anti-myc antibody and followed by Western blotting analysis. (C) Both the WWE and RING domains of RNF146 are required for PTEN degradation. 293T cells were transfected with constructs as described in B. Twenty-four hours after transfection, cells were treated with DMSO, 10 μM MG132, or 5 μM XAV939 for 6 h. Cell lysates were examined by Western blotting using the indicated antibodies. (D) Overexpression of RNF146, but not of the RNF146-△WWE mutant or the RNF146-△RING mutant, destabilizes PTEN. 293T transfection was conducted as described in B. Cells were treated with 100 μg/mL cycloheximide (CHX) for the indicated times. Protein levels were analyzed by immunoblotting using antibodies as indicated (left panel) and quantified (right panel). (E) RNF146 ubiquitinates PTEN in vivo. 293T cells were transfected with constructs encoding HA-tagged ubiquitin, GFP-tagged PTEN, and myc-tagged wild-type RNF146, the △WWE mutant of RNF146, or the △RING mutant of RNF146. Cells were treated with MG132 before being collected and analyzed by immunoprecipitation/Western blotting using the indicated antibodies. (F) Depletion of RNF146 stabilizes PTEN in vivo. RNF146 stable knockdown cells and control HCT116 cells were harvested, and cell lysates were examined by Western blotting and RT–PCR. (G) Knockdown of RNF146 suppresses PTEN ubiquitination in vivo. RNF146 stable knockdown cells and control cells were transfected with HA-ubiquitin and treated with 10 μM MG132 for 6 h. Cell lysates were immunoprecipitated with anti-HA antibody, and Western blotting was carried out using the indicated antibodies.