Abstract
For birds, unpredictable environments during the energetically stressful times of moulting and breeding are expected to have negative fitness effects. Detecting those effects however, might be difficult if individuals modulate their physiology and/or behaviours in ways to minimize short-term fitness costs. Corticosterone in feathers (CORTf) is thought to provide information on total baseline and stress-induced CORT levels at moulting and is an integrated measure of hypothalamic–pituitary–adrenal activity during the time feathers are grown. We predicted that CORTf levels in northern common eider females would relate to subsequent body condition, reproductive success and survival, in a population of eiders nesting in the eastern Canadian Arctic during a capricious period marked by annual avian cholera outbreaks. We collected CORTf data from feathers grown during previous moult in autumn and data on phenology of subsequent reproduction and survival for 242 eider females over 5 years. Using path analyses, we detected a direct relationship between CORTf and arrival date and body condition the following year. CORTf also had negative indirect relationships with both eider reproductive success and survival of eiders during an avian cholera outbreak. This indirect effect was dramatic with a reduction of approximately 30% in subsequent survival of eiders during an avian cholera outbreak when mean CORTf increased by 1 standard deviation. This study highlights the importance of events or processes occurring during moult on subsequent expression of life-history traits and relation to individual fitness, and shows that information from non-destructive sampling of individuals can track carry-over effects across seasons.
Keywords: carry-over effects, moult, feather corticosterone, reproductive success, avian cholera, common eider
1. Introduction
To cope with unpredictable environments, individuals can modulate their physiology and behaviour to minimize short-term fitness costs. Although environmental factors can influence individual condition and fitness directly over the short term, they might be more likely to result in indirect consequences (carry-over effects, COEs) later in life [1,2]. COEs are defined as events or processes that occur in one season and that can affect an individual's performance in a subsequent period [2]. COEs on the state of individuals can have important repercussions by magnifying or reducing population regulatory processes [3,4]. For migratory species, obtaining relevant metrics of individual state outside the breeding period is challenging, which makes identifying linkages between conditions experienced at specific locations or habitats and phases of the life cycle also challenging, more so owing to the difficulty in tracking migrants across seasons and locations [2].
Studies of migratory bird species provide examples of COEs. In earlier studies, arrival date on the breeding grounds was shown to be related to factors that occurred prior to the breeding season such as use of high-quality versus marginal wintering habitats [5–7]. The importance of spring body condition on reproduction is another example of a COE reported in several income–capital breeders [8–11]. Recently, an experimental manipulation of greater snow geese (Anser caerulescens) showed that stressful events (captivity) during migration affected subsequent reproduction [12]. Despite these examples, COEs are understudied, particularly in the context of novel environmental challenges such as climate change, food web disruption or emerging infectious diseases. Such factors have the potential to either magnify or ameliorate COEs making detection and the subsequent study of COEs and their impacts context-dependent. Also important in the study of COEs is the ease of measurement and reliability of indices by which conditions experienced ‘earlier on’ are assessed and tracked.
In birds, corticosterone (CORT) is the primary glucocorticoid released by activation of the hypothalamic–pituitary–adrenal (HPA) axis in response to a stressor [13]. CORT is responsible for mediating allostasis and promoting foraging and gluconeogenesis [14]; high levels of CORT can be a consequence of exposure to a threat or a result of increasing requirements for energy, movement (locomotion) and/or metabolism [15–17]. The stress response and quantification of CORT in natural populations have become important components of many studies in ecology, physiology and conservation biology [18–22].
Stress hormone levels have been linked to body condition in several species, although the mechanisms are not clear [22]. In upland geese (Chloephaga picta leucoptera), individuals with higher faecal CORT levels had decreased body condition [23]. Experimentally, tree swallow (Tachycineta bicolor) nestlings that received CORT implants showed reduced growth rates compared to controls [24]. Since increased CORT may affect body condition, CORT levels may be linked to arrival date and reproductive success in subsequent seasons.
In birds, CORT is deposited into feathers during growth so that the amount of CORT measured in a feather can provide an index of an individual's HPA activity during the growth of that feather and provides an integrated measure of CORT [24–26]. Previous studies have demonstrated the utility of measuring CORT in feathers and have shown that feather CORT (CORTf) is related to parental efficiency [26], social signals [27], nest microclimate [28], egg mass [29], cost of reproduction [30] and possibly climatic conditions [31]. Thus, CORTf has the potential to be used as a reliable index to study COEs of events occurring during the moulting period on the subsequent breeding period. In Anatidae, including northern common eiders (Somateria mollissima borealis), all flight feathers are moulted simultaneously once a year in late summer, after breeding. A feather collected in spring, prior to breeding, could thus provide an indication of the energetic demands experienced by the moulting birds approximately nine months earlier.
In addition to increasing energetic and catabolic costs, elevated CORT can also alter feather quality. Elevated levels of CORT in passerines during moult can affect the rate of feather growth [13,24] and experimental increases in CORT resulted in a decline in feather quality [32]. If eiders with higher CORTf levels have diminished feather quality, this may lower their flight or foraging efficiency during the over-wintering period and result in negative COEs into the breeding season. In common eiders, CORT measured in feathers probably reflects responses to environmental conditions experienced by birds during moult [31] and may potentially be used as a metric to study COEs of responses to climatic conditions on subsequent reproduction and survival many months later. Furthermore, it may also be used as a tool to examine the relationship between glucocorticoid responses during the molting period and infectious diseases.
Glucocorticoid levels can affect susceptibility to disease in many species, usually through effects on immune function [33] and energy metabolism. Exposure to chronically elevated CORT levels may decrease immune function [34] and increase susceptibility to disease, and even acute stress has been shown to affect survival of eiders in the face of an infectious disease outbreak [35]. Avian cholera (caused by the bacterium Pasteurella multocida) has been a cause of massive annual adult mortality in common eiders nesting at our study site in the low Arctic since 2005 [35,36]. Female eiders do not eat during the approximately 26 day incubation period. Egg laying and incubation are energetically demanding activities that may reduce immune function and future fecundity [37]. Large clutch sizes in eiders are associated with lower survival of female eiders in the face of severe avian cholera outbreaks [36]. Prior to 2005, avian cholera had not been documented in this population of eiders [35], and avian cholera in northern common eiders in the eastern Canadian arctic has previously only been documented in northern Quebec (Canadian Wildlife Health Cooperative, S. Iverson, N. J. Harms 2012, unpublished data).
Breeding success of common eiders is strongly influenced by body condition at time of breeding and by timing of migration [38]. Here, we expand the previous path analyses of Descamps et al. [38] by testing whether HPA activity during moult could be carried-over approximately nine months later to affect the timing of migration and arrival condition, and have direct or indirect links to reproductive success and survival, in the face of avian cholera outbreaks. The unexpected appearance of annual disease outbreaks in our study colony was the impetus behind our investigation into the potential COEs of events occurring during moult on the following breeding season.
2. Material and methods
(a). Study area and field methods
Eiders were captured on Mitivik Island (64°02′ N, 81°47′ W) in the East Bay Migratory Bird Sanctuary, Nunavut, Canada, from 2007 to 2011 [31,32]. Eiders were captured using large mist nets very early in the season when they were flying over the colony; we therefore assumed that capture date was a good proxy of arrival date [39]. At capture, body mass was measured using a Pesola scale (±2.5 g), and one tail feather (second lateral right feather) was plucked from each individual and stored in an envelope in a dark and dry place until laboratory analyses. All eider flight feathers are moulted simultaneously once a year in late summer, after breeding. Commonly, eider tail feathers are grown during moult in August–September, following the breeding season [40] and prior to autumn migration. Eiders from the eastern Canadian Arctic winter along the western coast of Greenland and northeastern coast of Labrador, Canada [40,41].
Venous blood samples were collected from a subset of female eiders. Blood samples were collected from the tarsal vein within 3 min of capture, placed into heparinized vials and centrifuged to harvest plasma. Plasma samples were stored frozen at −20°C in the field and −80°C in the laboratory until analysis for CORT. Although the plasma was collected as part of another study, we used the plasma CORT data to examine the relationship between plasma baseline CORT levels and CORTf. Eiders were banded with a metal band (United States Geological Survey) and two coloured alphanumeric Darvic bands (Pro-Touch, Saskatoon, Canada) [35]. All females were also marked with a unique colour and shape combination of two temporary plastic nasal markers (Juno Inc., Minneapolis, MN, USA) to enable identification of individual birds from a distance. We attached nasal markers with synthetic absorbable suture monofilament (Polydiaxanone suture, 2.0 or 3.0 metric; Ethicon, Markham, Canada), so that nasal tags would be shed prior to autumn migration. We restricted the analyses to birds captured during the prelaying period to compare body condition and avoid any effect of egg laying on body mass. To do so, for each year, we included data only from birds caught before the date at which more than 2.5% of the population had started laying [31]. Individuals with known laying dates were subsequently added to the dataset if known laying date was later than capture date (with a buffer of 3 days to account for potential error on laying date estimation). Arrival and laying dates were standardized relative to the median (0—median arrival or laying date in each year). Because body mass alone is a better predictor of condition than mass corrected for body size in this species [38,39], body mass was used as our measure of condition.
In 2011, 69 female eiders received subcutaneous corticosterone or sham implants (Innovative Research of America, Sarasota, FL, USA) as part of a separate study, and 44 of the implanted birds were included in this study. Such manipulation could have induced changes in eider reproductive outcomes, which could alter our conclusions. We performed all analyses with and without inclusion of eiders captured in 2011 to assess the robustness of our conclusions. Precise information on reproduction (lay date of the first egg and hatching success—at least one duckling hatched) for all eiders in this study was collected by monitoring nesting birds from eight observation blinds strategically located within the colony. Observation blinds allowed us to monitor over 90% of the eider nesting area [35] while minimizing disturbance to the colony. Final number of ducklings hatched was available for a very limited number of females included in this study. Therefore, we did not examine the link between CORTf and number of ducklings.
The island was scanned with spotting scopes twice a day throughout the nesting season to detect nesting females. Females observed greater than or equal to 2 times at the same nest within 36 h were considered to be breeding, and nesting status and fate were monitored twice daily until hatch or nest failure [35]. We were able to evaluate the lay date and nesting success of up to 350 (marked and unmarked) females each year [36,42].
Carcasses of nasal-tagged female eiders that died on their nests were located and collected during daily observation periods, or were recovered at the end of the breeding season when transects spaced 1 m apart were walked across the entire island by five observers to enumerate dead birds. Following the avian cholera outbreak on East Bay Island in 2005 [36], we assumed that eiders found dead on their nests or at the end of the breeding season died due to avian cholera. A subset of eider carcasses recovered each year were submitted to the Canadian Wildlife Health Cooperative for confirmation of the diagnosis of avian cholera using gross and histopathologic findings and bacteriology [43]. Marked birds that were no longer observed on the colony and not found dead were assumed to have survived an avian cholera outbreak. This assumption is feasible because eiders that abandon their nests leave the colony within 24 h [42] and thus are no longer exposed to the disease during the avian cholera outbreak. Furthermore, given that the island is very small (24 ha), we are confident that our methods for monitoring nests and surveying transects are effective at detecting the majority of carcasses on the island.
(b). Corticosterone analysis
CORTf measurements were performed using a previously established protocol [25] that includes a methanol-based extraction followed by analysis of the extracts via radioimmunoassay. This method has been previously used for eider feathers [31] and other avian species [26,28,44,45]. In this study, we assessed the efficiency of methanol extraction by including eider feather samples spiked with a small amount (approx. 5000 CPM) of 3H-corticosterone in the extraction. Greater than 92% of the radioactivity was recoverable in the reconstituted samples. Bortolotti et al. [25,27] showed that CORT is deposited into feathers in a time-dependent fashion; therefore, our values are expressed as a function of feather length (pg mm−1). All samples were measured in duplicate and were run randomized and blind. Assay variability was determined as the per cent coefficient of variation (CV) resulting from repeated measurement of samples spiked with a known amount of CORT in each assay. The average within-assay variation was 5.4% (range 2–10%), and inter-assay variation was 13.7%. Serial dilution of feather extracts from eider feathers produced displacement curves that were parallel to the standard curves. Hormone analyses were performed at the Department of Biology, University of Saskatchewan (Canada).
Baseline plasma CORT was analysed using a previously validated enzyme-linked immunoassay (Assay Designs, Ann Arbor, MI, USA; [46]) run in triplicate at a 1 : 20 dilution with 1.5% of kit-provided steroid displacement buffer. Each plate was run with a kit-provided standard curve by serially diluting a 200 000 pg ml−1 CORT standard and a control of laying hen plasma (Sigma-Aldrich Canada, Oakville, Ontario, Canada). Assay plates were read on a plate reader at a wavelength of 405 nM, and the mean inter- and intra-assay CV across all plates was 7.17% and 6.22%, respectively.
(c). Path analyses
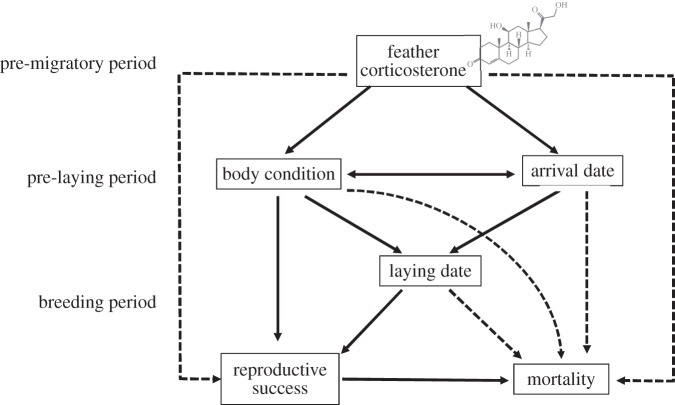
The importance of HPA activity levels during pre-migratory moulting period on reproduction and survival was assessed using path analysis, a special form of structural equation model [47], following Shipley [48]. The principle of the method is to specify how the variables are linked together in terms of direct and indirect effects or relationships. Information on CORTf, condition at arrival, arrival date, reproduction (laying date, reproductive success) and survival was available for 242 eider females from 2007 to 2011 (table 1). Among the 242 females, only two females were sampled in 2 years representing less than 1% of the data. The two additional measurements of the same individuals were considered as being independent. We developed our path diagram (figure 1) from a similar analysis that tested a condition-dependent optimization model on the same eider population [38]. We expanded the relationships (both direct and indirect) to include CORTf, reproductive success and survival. Arrows in figure 1 indicate relationships between two variables following event chronology (moulting, pre-breeding and breeding). Arrival date and body condition were assessed at the same time so determining causation was not possible. Mortality of female eiders due to avian cholera was detected following nest initiation [35]. Therefore, we investigated whether lay date or nest success could affect the survival of eiders during an avian cholera outbreak.
Table 1.
Description of model variables by year for female northern common eiders captured on East Bay Island, Nunavut, Canada.
| year | n common eiders | CORTf (pg mm−1) (mean ± s.d.) | body condition (kg) (mean ± s.d.) | Julian arrival date (mean ± s.d.) | Julian lay date (mean ± s.d.) | reproductive success (%) | % eiders survived outbreak |
|---|---|---|---|---|---|---|---|
| 2007 | 106 | 5.44 ± 2.00 | 2.191 ± 0.165 | 172.7 ± 3.2 | 183.7 ± 5.2 | 42.5 | 83 |
| 2008 | 38 | 4.33 ± 1.23 | 2.218 ± 0.132 | 171.2 ± 3.3 | 179.9 ± 5.5 | 44.7 | 73.7 |
| 2009 | 44 | 4.05 ± 1.23 | 2.225 ± 0.167 | 176.4 ± 4.2 | 184.8 ± 5.7 | 20.5 | 95.5 |
| 2010 | 10 | 3.58 ± 0.97 | 2.213 ± 0.164 | 166.4 ± 4.9 | 176.6 ± 5.2 | 50.0 | 100.0 |
| 2011 | 44 | 5.82 ± 1.61 | 2.255 ± 0.206 | 170.0 ± 2.5 | 179.8 ± 4.5 | 50.0 | 100.0 |
Figure 1.
The hypothesized causal structure linking energetic management, as represented by CORTf, during molt, arrival state, timing of reproduction and fitness components (reproductive success and survival of an avian cholera outbreak) in the face of an avian cholera outbreak in an eider colony on East Bay Island, Nunavut, Canada. Solid lines indicate the significant paths included in the final best-fit model, while dotted lines indicate additional or alternative paths that were tested but not found significant. The direction of the arrows indicates predicted effect.
The fit of a generalized multi-level path model was assessed using the concept of d-sep (directional separation) tests [47]. A d-sep test represents a test of the statistical independence between two variables. If two variables are d-separated relative to a set of variables Z in a directed graph, then they are independent conditional on Z in all probability distributions such a graph can represent (see Shipley [48] for more details). Shipley [47] shows that for each acyclic path model, there is a subset of independence tests referred to as a ‘minimum basis set’ that account for all possible independence relationships (or claims). Model fit is evaluated using a set of (k) mutually independent claims of probabilistic independence that must be true if the structure of the hypothesized path model is correct. The null probabilities (pi) from these k-tests are used to derive Fisher's C statistic: C = −2∑ln(pi), which follows a χ2-distribution with 2k degrees of freedom [47]. The null hypothesis is that the proposed correlational structure of the model does not differ from the observed correlational structure in the data, and therefore p ≤ 0.05 indicates the proposed causal structure is incorrect [47]. To calculate path coefficients, each variable was standardized ((value – average)/s.d.) such that path coefficients represent standardized partial regression coefficients, or the standard deviation change in y when x is increased or decreased by 1 s.d. [47].
Shipley [48] showed how the d-sep test can be combined with generalized linear mixed models. We followed detailed instructions provided in Shipley [48] using the packages nlme and lme4 in R [49]. We used linear mixed models (using terms CORTf, arrival date, body condition, laying date, hatching success and survival) to regress each variable on its direct causes. A random Year effect was included in each model. The random effect accounted from 0.46 to 5.71% of the deviance explained depending on the dependent variable considered.
3. Results
The correlational structure of our path model (figure 2) was consistent with the correlational structure of the data (seven tests of probabilistic independence; Fisher's C14 = 8.82, p = 0.84; implied independencies did not differ from those observed). The model defined in figure 1 provided a strong fit to the data as indicated by the high p-values (null probabilities) of the goodness-of-fit tests (table 2). Partial regression slope of CORTf was not different from zero in all claims revealing no direct effect of CORTf on lay date, reproductive success or survival. CORTf was significantly different among years (F4,237 = 11.43; p < 0.001). Arrival date on the breeding colony was positively associated with CORTf (figure 3a; β = 0.26 ± 0.13 (s.e.); t236 = 2.07; p = 0.04). Lower body condition (body mass) at arrival was related to higher levels of CORTf (figure 3b; β = −22.56 ± 6.09; t236 = −3.71; p < 0.001). There was no direct relationship between CORTf and lay date (t236 = 1.50; p = 0.14), reproductive success (z236 = −1.38; p = 0.17) or survival eiders during the avian cholera outbreak (z236 = −0.16; p = 0.87). We found that body condition increased over time during the pre-breeding period (β = 6.78 ± 3.14; t236 = 2.16; p = 0.03), so that birds arriving later were in better body condition. As expected, arrival date was positively linked to lay date and birds that arrived earlier laid earlier, and eiders that arrived in better body condition also laid earlier (β = 0.73 ± 0.09; t235 = 8.52; p < 0.001 and β = −4.73 ± 1.75; t235 = −2.71; p = 0.007, respectively). Later lay date had a direct negative effect on reproductive success, so that birds laying earlier in the season were more likely to hatch at least one egg (β = −0.24 ± 0.04; z236 = −6.42; p < 0.001).
Figure 2.
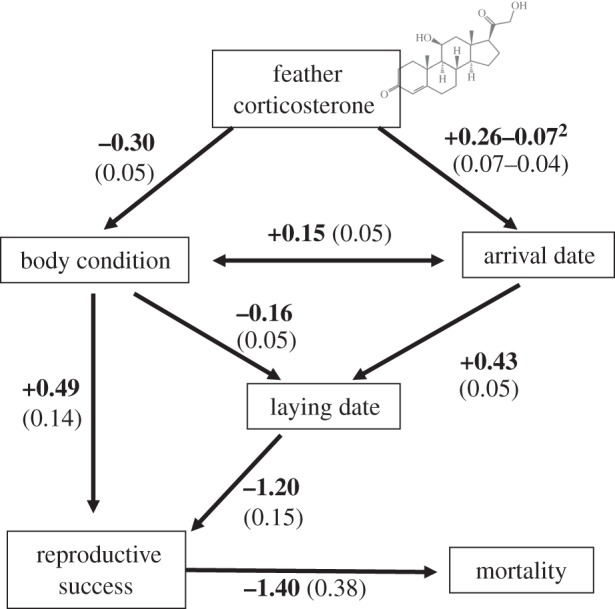
Standardized path coefficients in hypothesized structural model. Bold numbers are standardized beta coefficients with s.e in brackets (n = 242).
Table 2.
Test of conditional independence implied by the path diagram (figure 1). ((X; Y)|{Z} means that variables X and Y are independent conditional of variable Z (i.e. if Z is held constant, variation in X does not imply variation in Y). The associated mixed model used to test the independence claims are Y ∼ Z + X + 1|Year, where Year represents a random effect. The variable whose partial regression includes zero is X1 for all claims. Variables: X1 (CORTf), X2 (Arrival date), X3 (Body condition), X4 (Laying date), X5 (Hatching success) and X6 (Survival of an avian cholera outbreak).)
| d-sep claim of independence | mixed model | partial regression slope (s.e.) | null probability | (distribution) |
|---|---|---|---|---|
| (X1,X4)|{X2,X3} | X4 ∼ X1 + X2 + X3 + (1|Year) | 0.072 (0.176) | 0.68 | (normal) |
| (X1,X5)|{X4} | X5 ∼ X4 + X1 + (1|Year) | −0.046 (0.090) | 0.61 | (binomial) |
| (X2,X5)|{X1,X4} | X5 ∼ X4 + X1 + X2 + (1|Year) | 0.014 (0.096) | 0.89 | (binomial) |
| (X1,X6)|{X4} | X6 ∼ X4 + X1 + (1|Year) | 0.014 (0.039) | 0.71 | (binomial) |
| (X2,X6)|{X1,X4} | X6 ∼ X4 + X1 + X2 + (1|Year) | −0.074 (0.069) | 0.28 | (binomial) |
| (X3,X6)|{X1,X4} | X6 ∼ X4 + X1 + X3 + (1|Year) | 0.010 (0.039) | 0.79 | (binomial) |
| (X4,X6)|{X5} | X6 ∼ X4 + X5 + (1|Year) | 0.057 (0.046) | 0.21 | (binomial) |
Figure 3.
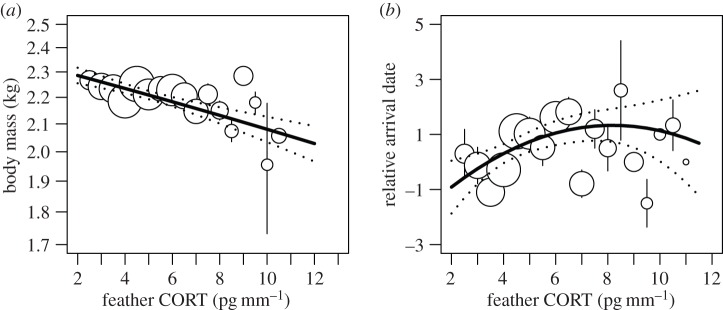
Relationship between feather corticosterone (CORTf), arrival date (a) and body condition (b) in female eiders. The model presented here controls for the other covariates (either mean arrival date or condition). Mean and s.e. are provided. Dot sizes are proportional to log (n). The fitted mixed linear model (black line) and its confidence interval at 95% (grey line) are shown.
Body condition at arrival was positively associated with reproductive success (β = 2.68 ± 0.90; z236 = 2.96; p = 0.003) but was not associated with survival. However, there was a direct negative relationship between reproductive success and survival (β = 1.29 ± 0.50; z242 = 2.59; p = 0.01) where birds that reproduced successfully were more likely to survive. The overall indirect relationships between CORTf and reproductive success and survival were relatively high; the sum of all products of path coefficients for each variable was −0.22 and −0.27, respectively. This indicates that if CORTf increased by 1 s.d. from its mean, reproductive success was decreased by 0.22 s.d. and survival of eiders during avian cholera outbreak decreased by 0.27 s.d. from its own mean. Female eiders captured in 2011 underwent an additional manipulation (subcutaneous CORT implant; see Material and methods) that was not done in any of the other years. Similar results were found when excluding eiders captured in 2011 from the analyses. The results presented in the manuscript thus included 2011 to maximize sample size. Within same individuals, there was no significant relationship between baseline CORT levels (measured in plasma during the pre-breeding period (O. P. Love, H. Hennin, H. G. Gilchrist, J. Bety 2006–2013, unpublished data) and feather CORT (F1,195 = 0.02; p = 0.89).
4. Discussion
Our results provide evidence that energetic management during the moulting period, reflected by CORT levels in feathers, can be carried over to the subsequent breeding season and affect reproductive success and survival. Using path analyses, we detected a direct relationship between CORTf levels during moult and body condition and arrival date the following year, and an indirect negative relationship between reproductive success and survival. The magnitude of the indirect relationship between CORTf and fitness parameters was important, with a decrease of approximately 0.25 s.d. from the mean of reproductive success and survival for every increase of 1 s.d. of CORTf. The importance of arrival date and condition on reproductive success was expected given that the path analysis developed here is an extension of Descamps et al. [38], who found similar relationships in accordance with the condition-dependent optimization model [9,50]. As per causal pathways drawn from the optimization model [38,50] birds can adjust their reproductive decisions as a function of their arrival date and body condition at arrival. Our study is probably unique in showing that both these variables can be significantly related to CORTf levels, which may reflect a level of energetic management experienced during the previous year. Increases in energetic challenges or response to stressors experienced during the time of moult in autumn can ultimately have significant fitness consequences, indirectly affecting both reproduction and survival in the following breeding season.
Another possible explanation is that CORTf values reflect basal CORT levels of individuals regardless of the time period considered. This hypothesis would gain credence if baseline CORT during the breeding or pre-breeding period could predict relative fitness of individuals [51–54]. However, we found no evidence for any relationship between CORTf and pre-breeding basal CORT within individuals, providing little support for this explanation. Furthermore, we also found that CORTf was not repeatable within individuals from year to year and was influenced more by environmental factors encountered during moult (e.g. temperature) rather than intrinsic measures of quality (e.g. body size) [31].
While COEs are increasingly reported to influence fitness components [2], measuring such effects at the individual level are rare in the literature, as is their measurement relative to novel environmental challenges. Furthermore, very few studies have reported events occurring during the previous autumn with latent effects on subsequent summer reproduction and/or survival. Interestingly, in an Icelandic eider colony, Jónsson et al. [55] reported negative effects of warm and wet autumns on subsequent clutch size. The authors argued that the effect was probably due to delay in migratory movements and in pair formation. In addition, we recently reported that warmer autumn temperatures were linked to slightly higher CORTf levels, suggesting a physiological cost to increasing temperatures in an arctic environment [31].
Finally, we documented that survival on the breeding colony was also indirectly related to increasing energetic demands experienced during moult the previous autumn (i.e. CORTf). Carry-over effects on survival or mortality rates from events occurring in autumn have been rarely reported in the literature [7,56,57]. Recently, Koren et al. [58] found that high CORTf levels in house sparrows (Passer domesticus) were predictive of lower survival over the subsequent winter. Crossin et al. [30] measured feather CORT in giant petrels (Macronectes spp.), which begin moult during the breeding season, and found that variation in CORTf was linked to both current reproductive success and future reproductive effort. In contrast to the results found in sparrows [58], CORTf levels in giant petrels were not related to overwinter survival [30]. Female petrels with high CORTf values were, however, successful breeders in the current year but more likely to defer breeding in the next year, suggesting that CORT upregulation does exact a cost on future effort [30]. Even if the mechanism remains unclear, energetic costs associated with maintaining elevated CORT levels have often been reported to explain reduced survival rates [20,21,59–61]. Since pre-breeding condition and CORTf were related in female eiders, such increased energy expenditure could be involved. However, survival was only indirectly linked to CORTf, through its positive association with breeding success. This agrees with previous studies from our research group [36,62] suggesting that breeding decision, reproductive investment and the duration of exposure to disease at the nesting colony are key factors explaining survival of eiders facing avian cholera outbreaks.
5. Conclusion
Our work emphasizes the importance of determining how events are linked throughout the annual cycle to better understand population dynamics of migratory animals. Our approach also highlights the importance of energetic management challenges outside the breeding period (possibly generated by climatic variability) that can have subsequent carry-over effects on reproduction and survival during outbreaks of avian cholera, an emerging disease in arctic-nesting common eiders. Little is known about the moulting period for many bird species, including eiders [63], thus our results shed some light on a relatively unknown stage of the annual cycle. Combining information that can be gained from non-destructively sampling a single feather, including stable isotopes [64–66], coloration [5] or physiological analysis such as hormone levels [58] can contribute to tracking COEs across seasons. Furthermore, considering both direct and indirect pathways may be required to understand relationships among spatio-temporally distinct events affecting individual fitness.
Acknowledgements
Many thanks to H. Hennin, H. Remenda, W. van Dijk, S. Cabezas, V. Fachal, J. McLeod and T. Marchant for assistance in the laboratory, and thanks to the numerous students and researchers, who assisted us in the field from 2007 to 2011. We thank S. Descamps, R. G. Clark, N. G. Yoccoz and an anonymous reviewer, and G. Fairhurst for helpful comments on the methods and manuscript.
Ethics statement
This study adhered to guidelines of the Canadian Council on Animal Care, and all protocols were reviewed and approved by the University Committee on Animal Care and Supply—Animal Research Ethics Board of the University of Saskatchewan (Protocol no. 20100063 to C.S.), the University Committee on Animal Care of the University of Windsor (Protocol no. 11–06 to O.P.L.), Environment Canada's Animal Care Committee (Protocol no.: EC-PN-07–008 (2007), EC-PN-08–026 to EC-PN-11–026 (2008–2011) to H.G.G.).
Data accessibility
Common eider body condition, arrival date, body condition, feather corticosterone, survival and reproductive data: Dryad doi.org/10.5061/dryad.rp30d.
Funding statement
Funding for this work was provided by the National Science and Engineering Council of Canada, Environment Canada, Duck Unlimited Institute for Wetland and Waterfowl Research, the University of Saskatchewan's Wildlife Health Research Fund, the Nunavut Wildlife Management Board, and Strategic Applications of Genomics in the Environment. N.J.H. was supported by a University of Saskatchewan's Interprovincial Graduate Fellowship, a Doctoral Award for Northern Research from the W. Garfield Weston Foundation, the Arctic Institute of North America's Jennifer Robinson Memorial Scholarship and the Lorraine Allison Memorial Scholarship and grants from the Northern Scientific Training Program.
References
- 1.Newton I. 2006. Can conditions experienced during migration limit the population levels of birds? J. Ornithol. 147, 146–166. (doi:1007/s10336-006-0058-4) [Google Scholar]
- 2.Harrison XA, Blount JD, Norris DR, Bearhop S. 2011. Carry over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 3.Norris DR. 2005. Carry over effects and habitat quality in migratory populations. Oikos 109, 178–186. ( 10.1111/j.0030-1299.2005.13671.x) [DOI] [Google Scholar]
- 4.Norris DR, Taylor CM. 2006. Predicting the consequences of carry-over effects for migratory populations. Biol. Lett. 2, 148–151. ( 10.1098/rsbl.2005.0397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliff LM. 2004. Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc. R. Soc. B 271, 59–64. ( 10.1098/rspb.2003.2569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Runge MC, Marra PP. 2005. Modelling seasonal interactions in the populations dynamics of migratory birds. In Birds of two worlds: the ecology and evolution of migration (eds Greenberg R, Marra PP.), pp. 375–389. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 7.Studds EC, Marra PP. 2005. Nonbreeding habitat occupancy and population processes: an upgrade experiment with a migratory bird. Ecology 86, 2380–2385. ( 10.1890/04-1145) [DOI] [Google Scholar]
- 8.Alisauskas RT. 2002. Arctic climate, spring nutrition, and recruitment in midcontinent lesser snow geese. J. Wildl. Manage. 66, 181–193. ( 10.2307/3802884) [DOI] [Google Scholar]
- 9.Bêty J, Gauthier G, Giroux JF. 2003. Body condition, migration, and timing of reproduction in snow geese: a test of the condition-dependent model of optimal clutch-size. Am. Nat. 162, 110–121. ( 10.1086/375680) [DOI] [PubMed] [Google Scholar]
- 10.Klaassen M, Bauer S, Madsen J, Ingunn T. 2006. Modelling behavioural and fitness consequences of disturbance for geese along their spring flyway. J. Appl. Ecol. 43, 92–100. ( 10.1111/j.1365-2664.2005.01109.x) [DOI] [Google Scholar]
- 11.Sedinger JS, Schamber JL, Ward DH, Nicolai CA, Conant B. 2011. Carry-over effects associated with winter location affect fitness, social status, and population dynamics in a long-distance migrant. Am. Nat. 178, E110–E123. ( 10.1086/662165) [DOI] [PubMed] [Google Scholar]
- 12.Legagneux P, Fast PLF, Guathier G, Bety J. 2012. Manipulating individual state during migration provides evidence for carry-over effects modulated by environmental conditions. Proc. R. Soc. B 279, 876–883. ( 10.1098/rspb.2011.1351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero LM, Strochlic D, Wingfield JC. 2005. Corticosterone inhibits feather growth: potential mechanism explaining seasonal downregulation of corticosterone during moult. Comp. Biochem. 142, 65–73. ( 10.1016/j.cbpa.2005.07.014) [DOI] [PubMed] [Google Scholar]
- 14.McEwan BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. ( 10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 15.Romero LM. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 251–255. ( 10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 16.Angelier F, Wingfield JC, Weimerskirch FH, Chastel O. 2010. Hormonal correlates of individual quality in a long-lived bird: a test of the ‘corticosterone–fitness hypothesis’. Biol. Lett. 6, 846–849. ( 10.1098/rsbl.2010.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fokidis B, Hurley L, Rogowski C, Sweazea K, Deviche P. 2011. Effects of captivity and body condition on plasma corticosterone, locomotor behaviour, and plasma metabolites in curve-billed thrashers. Phys. B. Zool. 84, 595–606. ( 10.1086/662068) [DOI] [PubMed] [Google Scholar]
- 18.Wasser SK, Bevis K, King G, Hanson E. 1997. Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv. Biol. 11, 1019–1022. ( 10.1046/j.1523-1739.1997.96240.x) [DOI] [Google Scholar]
- 19.Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24. ( 10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 20.Wikelski M, Cooke SJ. 2006. Conservation physiology. Trends Ecol. Evol. 21, 38–46. ( 10.1016/j.tree.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 21.Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA. 2007. Stress response during development predicts fitness in a wild long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884. ( 10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husak JF, Moore IT. 2008. Stress hormones and mate choice. Trends. Ecol. Evol. 23, 532–534. ( 10.1016/j.tree.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 23.Gladbach A, Gladbach DJ, Koch M, Kuchar A, Möstl E, Quillfeldt P. 2011. Can faecal glucocorticoid metabolites be used to monitor body condition in wild Upland geese (Chloephaga picta leucoptera)? Behav. Ecol. Sociol. 65, 1491–1498. ( 10.1007/s00265-011-1169-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairhurst GD, Marchant T, Soos C, Machin K, Clark RG. 2013. Experimental relationships between levels of corticosterone in plasma and feathers in a free-living bird. J. Exp. Biol. 216, 4071–4081. () [DOI] [PubMed] [Google Scholar]
- 25.Bortolotti GR, Marchant TA, Blas J, German T. 2008. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Func. Ecol. 22, 494–500. ( 10.1111/j.1365-2435.2008.01387.x) [DOI] [Google Scholar]
- 26.Fairhurst GD, Navarro J, González-Solís J, Marchant TA, Bortolotti GR. 2012. Feather corticosterone of a nestling seabird reveals consequences of sex-specific parental investment. Proc. R. Soc. B 279, 177–184. ( 10.1098/rspb.2011.0884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bortolotti GR, Mougeot F, Martinez-Padilla J, Webster LMI, Piertney SB. 2009. Physiological stress mediates the honesty of social signals. PLoS ONE 4, e4983 ( 10.1371/journal.pone.0004983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairhurst GD, Treen GD, Clark RG, Bortolotti GR. 2012. Nestling corticosterone response to microclimate in an altricial bird. Can. J. Zool. 90, 1422–1430. ( 10.1139/cjz-2012-0096) [DOI] [Google Scholar]
- 29.Kouwenberg A, Hipfner JM, McKary DW, Storey AE. 2013. Corticosterone and stable isotopes in feathers predict egg size in Atlantic puffins Fratercula arctica. Ibis 155, 413–418. ( 10.1111/ibi.12030) [DOI] [Google Scholar]
- 30.Crossin GT, Phillips RA, Lattin CR, Romero LM, Williams TD. 2013. Corticosterone mediated costs of reproduction link current to future breeding. Gen. Comp. Endocrin. 193, 112–120. ( 10.1016/j.ygcen.2013.07.011) [DOI] [PubMed] [Google Scholar]
- 31.Legagneux PL, Harms NJ, Gauthier G, Chastel O, Gilchrist GL, Bortolotti G, Bêty J, Soos C. 2013. Does feather corticosterone reflect individual quality or external stress in arctic-nesting migratory birds? PLoS ONE 8, e82644 ( 10.1371/journal.pone.0082644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lattin CR, Reed JM, DeRochers DW, Romero LM. 2011. Elevated corticosterone correlates with corticosterone-induced decreased feather quality: a validation study. J. Avian Biol. 42, 247–252. ( 10.1111/j.1600-048X.2010.05310.x) [DOI] [Google Scholar]
- 33.Bourgeon S, Raclot T. 2006. Corticosterone selectively decreases humoral immunity in female eiders during incubation. J. Exp. Biol. 209, 4957–4965. ( 10.1242/jeb.02610) [DOI] [PubMed] [Google Scholar]
- 34.McEwen CA, et al. 1997. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Brain Res. Rev. 1–2, 79–133. ( 10.1016/S0165-0173(96)00012-4) [DOI] [PubMed] [Google Scholar]
- 35.Buttler IE, Gilchrist HG, Descamps S, Forbes MR, Soos C. 2011. Handling stress of female common eiders during avian cholera outbreaks. J. Wildl. Manage. 75, 283–288. ( 10.1002/jwmg.38) [DOI] [Google Scholar]
- 36.Descamps S, Gilchrist HG, Bêty J, Buttler EI, Forbes MR. 2009. Costs of reproduction in a long-lived bird: large clutch size is associated with low survival in the presence of a highly virulent disease. Biol. Lett. 5, 278–281. ( 10.1098/rsbl.2008.0704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanssen SA, Hasselquist D, Folstad I, Erikstad KE. 2005. Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc. R. Soc. B 272, 1039–1046. ( 10.1098/rspb.2005.3057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Descamps S, Bêty J, Love OP, Gilchrist HG. 2011. Individual optimization of reproduction in a long-lived migratory bird: a test of the condition-dependent model of laying date and clutch size. Func. Ecol. 25, 671–681. ( 10.1111/j.1365-2435.2010.01824.x) [DOI] [Google Scholar]
- 39.Descamps S, Yoccoz NG, Gaillard J-M, Gilchrist HG, Erikstad KE, Hanssen SA, Cazelles B, Forbes MR, Bêty J. 2010. Detecting population heterogeneity in effects of North Atlantic Oscillations on seabird body condition: get into the rhythm. Oikos 119, 1526–1536. ( 10.1111/j.1600-0706.2010.18508.x) [DOI] [Google Scholar]
- 40.Mosbech A, Gilchrist G, Merkel F, Sonne C, Flagstad A, Nyegaard H. 2006. Year-round movements of northern common eiders Somateria mollissima borealis breeding in Arctic Canada and West Greenland followed by satellite telemetry. Ardea 94, 651–665. [Google Scholar]
- 41.Goudie RI, Robertson GJ, Reed A. 2000. Common eider (Somateria mollissima). In The birds of North America, no. 546 (eds Poole A, Gill F.). Philadelphia, PA: American Ornithologist's Union and Academy of Natural Sciences of Philadelphia. [Google Scholar]
- 42.McKinnon L, Gilchrist HG, Scribner KT. 2006. Genetic evidence for kin-based social structure in common eiders (Somateria mollissima). Behav. Ecol. 17, 614–621. ( 10.1093/beheco/ark002) [DOI] [Google Scholar]
- 43.Legagneux P, et al. 2014. No selection on immunological markers in response to a highly virulent pathogen in an Arctic breeding bird. Evol. Appl. 7, 765–773. ( 10.1111/eva.12180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harms NJ, Fairhurst GD, Bortolotti GR, Smits JEG. 2010. Variation in immune function, body condition, and feather corticosterone in nestling tree swallows (Tachycineta bicolor) on reclaimed wetlands in the Athabasca oil sands, Alberta, Canada. Environ. Pollut. 158, 841–848. ( 10.1016/j.envpol.2009.09.025) [DOI] [PubMed] [Google Scholar]
- 45.Fairhurst GD, Frey MD, Reichert JF, Szelest I, Kelly DM, Bortolotti GR. 2011. Does environmental enrichement reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS ONE 6, e17663 ( 10.1371/journal.pone.0017663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Love OP, Williams TD. 2008. Plasticity in the adrenalcortical response of a free-living vertebrae: the role of pre- and post-natal development stress. Horm. Behav. 54, 496–505. ( 10.1016/j.yhbeh.2008.01.006) [DOI] [PubMed] [Google Scholar]
- 47.Shipley B. 2000. Cause and correlation in biology: a user's guide to path analysis, structural equations, and causal inference, p. 317 New York, NY: Cambridge University Press. [Google Scholar]
- 48.Shipley B. 2009. Confirmatory path analysis in a generalized multi-level context. Ecology 90, 363–368. ( 10.1890/08-1034.1) [DOI] [PubMed] [Google Scholar]
- 49.R Development Core Team 2013. R studio v 3.1, Boston, MA: Free Software Foundation. [Google Scholar]
- 50.Rowe L, Ludwig D, Schluter D. 1994. Time, condition, and the seasonal decline on avian clutch size. Am. Nat. 143, 698–722. ( 10.1086/285627) [DOI] [Google Scholar]
- 51.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. ( 10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 52.Cockrem J. 2013. Corticosterone responses and personality in birds: individual variation and the ability to cope with environmental changes due to climate change. Gen. Comp. Endocrin. 190, 156–163. ( 10.1016/j.ygcen.2013.02.021) [DOI] [PubMed] [Google Scholar]
- 53.Schmid B, Tam-Danford L, Jenni-Eirmann S, Arlettaz R, Schaub M, Jenni L. 2013. Modulation of the adrenocortical response to acute stress with respect to brood value, reproductive success and survival in the Eurasian hoopoe. Oecologia 173, 33–44. ( 10.1007/s00442-013-2598-7) [DOI] [PubMed] [Google Scholar]
- 54.Narayan EJ, Cockrem J, Hero JM. 2013. Repeatability of baseline corticosterone and short-term corticosterone stress responses, and their correlation with testosterone and body condition in a terrestrial breeding anuran (Platymantis vitiana). Comp. B Phys. Mol. Integ. Phys. 165, 304–312. ( 10.1016/j.cbpa.2013.03.033) [DOI] [PubMed] [Google Scholar]
- 55.Jonsson JE, Gardarsson A, Gill JA, Petersen A, Gunnarsson TG. 2009. Seasonal weather effects on the common eider, a subarctic capital breeder, in Iceland over 55 years. Clim. Res. 38, 237–248. ( 10.3354/cr00790) [DOI] [Google Scholar]
- 56.Dawson A, Hinsley SA, Ferns PN, Bonser RHC, Eccleston L. 2000. Rate of moult affects feather quality: a mechanism linking current reproductive effort to future survival. Proc. R. Soc. Lond. B 267, 2093–2098. ( 10.1098/rspb.2000.1254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell GW, Guglielmo CG, Wheelwright NT, Freeman-Gallant CR, Norris DR. 2011. Early life events carry over to influence pre-migratory condition in a free-living songbird. PLoS ONE 6, e28838 ( 10.1371/journal.pone.0028838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koren L, Nakagawa S, Burke T, Soma KK, Wynne-Edwards KE, Geffen E. 2012. Non-breeding feather concentrations of testosterone, corticosterone and cortisol are associated with subsequent survival in wild house sparrows. Proc. R. Soc. B 279, 1560–1566. ( 10.1098/rspb.2011.2062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDougall-Shakleton SA, Dindia L, Newman AEM, Potvin D, Steart KA, MacDougall-Shackleton EA. 2009. Stress, song and survival in sparrows. Biol. Lett. 5, 746–748. ( 10.1098/rsbl.2009.0382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212. ( 10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goutte A, Antoine E, Weimerskirch H, Chastel O. 2010. Age and the timing of breeding in a long-lived bird: a role for stress hormones? Func. Ecol. 24, 1007–1016. ( 10.1111/j.1365-2435.2010.01712.x) [DOI] [Google Scholar]
- 62.Descamps S, Jenouvrier S, Gilchrist HG, Forbes M. 2012. Avian cholera, a threat to the viability of an arctic seabird colony? PLoS ONE 7, e29659 ( 10.1371/journal.pone.0029659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savard JP, Lesage L, Gilliland S, Gilchrist HG, Giroux JF. 2011. Molting, staging, and wintering locations of common eiders breeding in the Gyrfalcon Archipelago, Ungava Bay. Arctic 64, 197–206. ( 10.14430/arctic4099) [DOI] [Google Scholar]
- 64.Marra PP, Hobson KA, Holmes RT. 1998. Linking winter and summer events in a migratory bird by using stable carbon isotopes. Science 282, 1884–1886. ( 10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 65.Sorensen MC, Hipfner JM, Kyser TK, Norris RD. 2009. Carry-over effects in a Pacific seabird: stable isotope evidence that pre-breeding diet influences reproductive success. J. Anim. Ecol. 78, 460–467. ( 10.1111/j.1365-2656.2008.01492.x) [DOI] [PubMed] [Google Scholar]
- 66.Rushing CS, Ryder TB, Saracco JF, Marra PP. 2014. Assessing migratory connectivity for a long-distance migratory bird using multiple intrinsic makers. Ecol. App. 24, 445–456. ( 10.1890/13-1091.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Common eider body condition, arrival date, body condition, feather corticosterone, survival and reproductive data: Dryad doi.org/10.5061/dryad.rp30d.