Summary
Fragile X Syndrome (FXS) is a trinucleotide repeat disorder that results in the silencing of the Fragile X Mental Retardation 1 gene (FMR1), leading to a lack of the FMR1 protein (FMRP). FMRP is an mRNA-binding protein that regulates the translation of hundreds of mRNAs important for synaptic plasticity. Several of these pathways have been identified and have guided the development of targeted treatments for FXS. Here we present evidence that serotonin is dysregulated in FXS and treatment with the selective serotonin reuptake inhibitor (SSRI) sertraline may be beneficial for individuals with FXS, particularly in early childhood.
Keywords: Fragile X Syndrome, fragile X mental retardation protein, selective serotonin reuptake inhibitors, sertraline
1. Introduction
Fragile X Syndrome (FXS) is the leading inherited cause of intellectual disability and autism. A hallmark feature of FXS is delay in receptive and expressive language development and this is often the presenting sign of FXS in early childhood (1,2). Symptoms of anxiety, attention deficit hyperactivity disorder (ADHD), and hyperarousal with sensory stimuli are all typical of children with FXS (3–8).
FXS is a monogenic disorder caused by an expanded CGG repeat in the 5′ untranslated region of the FMR1, located on the long arm of chromosome X (9). It is considered normal to have between 5–40 CGG repeats in FMR1. The premutation is characterized by 55 to 200 CGG repeats and a full mutation occurs at > 200 CGG repeats (10). In the full mutation, FMR1 becomes methylated, resulting in significantly reduced or absent levels of the FMR1 protein (FMRP). FMRP is a selective, inhibitory, mRNA-binding protein that regulates the translation of mRNAs into their respective proteins (11). It is expressed throughout the body, but is especially critical in neuronal soma and dendrites because most of the proteins that are regulated by FMRP are important for synaptic plasticity (12,13). Since FMRP expression depends on age, the lack of FMRP in FXS is particularly disruptive in early development, when synapse formation is especially dynamic (14).
As a result of the loss of FMRP expression, many neurochemical pathways are disrupted in patients with FXS (15,16). For example, there is up-regulation of the metabotropic glutamate receptor 5 (mGluR5) pathway leading to enhanced long term depression (LTD), down-regulation of GABA pathways (17), and dysregulation of dopamine and cholinergic pathways (12,18). Here we discuss evidence that serotonin (5-hydroxytryptamine, 5-HT) represents another potential target for mechanistic therapy.
2. Serotonin in FXS mouse models
Findings in animal models of FXS provide evidence that serotonin can be specifically helpful in treating the dysregulated pathways in FXS.
One of the pathways known to be dysregulated in FXS is the mGluR-regulated LTD pathway (11,19,20). In the mGluR-mediated LTD mechanism, stimulation of postsynaptic group 1 (Gp1) mGluRs at a dendrite rapidly evokes local protein synthesis that results in the internalization of AMPA receptors (AMPARs), such as GluA1 (GluR-A) from the synapse. Among the proteins synthesized upon stimulation at the dendrite is FMRP. FMRP is an mRNA translation repressor and serves as the negative feedback to the increased protein synthesis (20). Without FMRP, such as in FXS, evoked protein synthesis runs unchecked, leading to excessive AMPAR internalization and, thus, exaggerated LTD in response to a stimulus. Using hippocampal slices from the FXS mouse model, Costa et al. (21) showed that stimulation of postsynaptic 5-HT7 serotonin receptors successfully ameliorates the exaggerated mGluR5-mediated synaptic LTD in FXS to wild-type levels.
GluA1-dependent long-term potentiation (LTP) is also disrupted in FXS (22), and can be partly corrected by serotonin. Lim et al. (23) showed this with an experiment measuring synaptic GluA1 delivery in hippocampal slice preparations from Fmr1 knockout (KO) and wild-type mice. Although GluA1 delivery to the synapse is normally impaired in the FXS model, application of a 5HT2B-R agonist restored about 20% of GluA1 synaptic delivery.
3. Serotonin in patients with FXS and autism
There is limited research concerning serotonin levels in people with FXS. One study done by Hessl et al. (24) found that genetic polymorphisms in the gene encoding serotonin reuptake transporter protein correlated with levels of aggression in patients with FXS. Those individuals with polymorphisms conferring higher reuptake (a 44 base pair insertion in the promoter region at 17q11.2 of the 5-HTT receptor) correspond to a more aggressive FXS phenotype.
Although there have been few studies specifically in the FXS population, significant research has been done in children with autism. This research is still highly relevant since there is significant overlap between autism and FXS (3,25). An analysis of de novo gene mutations resulting in autism showed that 30–50% of autism genes are regulated by or associated with FMRP (26). As previously mentioned, FXS is the leading monogenetic cause of autism. One third of patients with FXS are diagnosed with autism and another third meet criteria for autism spectrum disorder (ASD) (25,27). Children with FXS that did not meet ASD criteria still had autistic features such as poor eye contact, hand flapping or hand stereotypies, in addition to shyness or social anxiety (5,27).
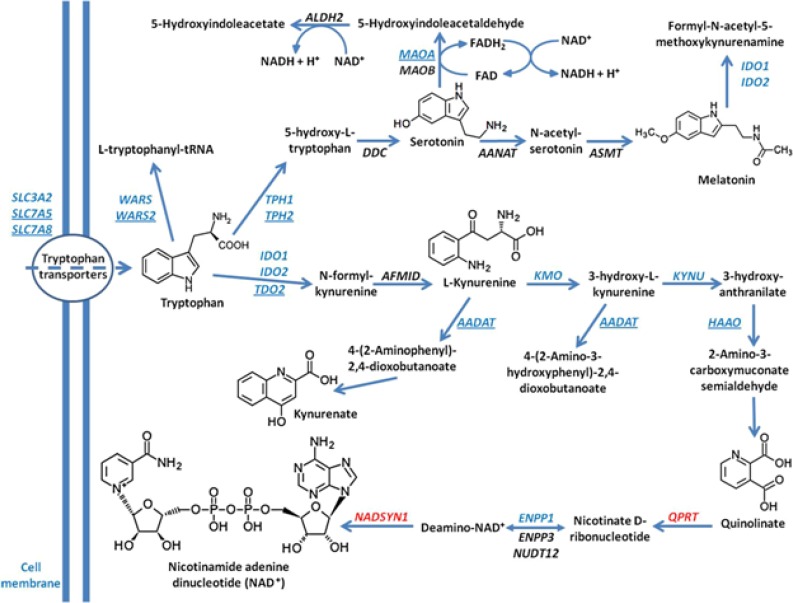
There is ample evidence that normal serotonin synthesis is disrupted in patients with autism. For example, it has been shown that metabolism of tryptophan, the amino acid precursor to serotonin, is decreased in patients with autism (28). Additionally, studies in which adults with autism were deprived of tryptophan found that this diet worsened autistic symptoms (29). Tryptophan metabolism occurs in mitochondria and follows one of two pathways, leading to either the creation of serotonin/melatonin or kynurenin-quinolinic acid. Both pathways also lead to nicotinamide adenine dinucleotide (NADH) production. In experiments done by Boccuto et al. (28), comparisons between lymphoblastoid cells from patients with autism and controls revealed a uniting abnormality in the cells from patients with autism: reduced ability to process tryptophan. The origin of autism in the study patients included both syndromal and non-syndromal cases. Subsequent genetic analysis revealed abnormally low levels of key enzymes involved in mitochondrial tryptophan metabolism (Figure 1). These proteins include SLCA5 and SLC7A8 (enzymes involved in tryptophan transport into mitochondria), WARS2 (tryptophanyl tRNA synthetase), TPH2 (tryptophan hydroxylase 2, rate-limiting enzyme in the serotonin/melatonin pathway inside mitochondria), as well as TDO2 (tryptophan 2,3-dioxygenase) and AADAT (aminoadipate aminotransferase), enzymes involved in the kynurenin-quinoloinic acid pathway. It is interesting to note that children with FXS often have sleeping difficulties (30), which may be related to dysfunction of melatonin due to ineffective tryptophan processing. Therefore, is not surprising that patients with FXS usually show improvements in their sleep patterns with melatonin treatment (31).
Figure 1.
Tryptophan pathways in patients with autism. The figure illustrates the main intracellular pathways involving tryptophan. The microarray dataset of Boccuto et al. 2013 (consisting of patients with autism) are in blue, genes with increased expression are in red. Genes with statistically significant reduction of expression in patients with autism are underlined. (Note: Figure reprinted and legend adapted from “Decreased tryptophan metabolism in patients with autism spectrum disorders” by Boccuto L, et al., 2013, Molecular autism, 4(1), page 7. Copyright 2013 by BioMed Central. Reprinted with permission.)
Children with ASD also have a significantly different capacity for serotonin production compared to children without ASD during development. Serotonin levels are normally relatively high in the developing brain compared to adults. This peak appears between ages 2–5 years, when brain serotonin synthesis capacity reaches twice the levels found in the adult brain (32). After age 5, synthesis ability declines until age 15, when it reaches adult levels. Children with autism, however, do not reach the same 2–5 year old peak. Instead, their serotonin levels increase slowly, resulting in a relatively low level during the 2–5 year old period and ending up at a higher level in adulthood (32). This suggests that therapeutic intervention with an SSRI may be more beneficial during this critical window in early childhood as opposed to later in life for children with autism, including those with FXS.
Children with ASD also display abnormal cortical asymmetry in serotonin synthesis capacity (33). Notably, the specific pattern of asymmetry correlates with symptom presentation. For example, children with decreased left-sided serotonin synthesis have a higher rate of language impairment.
4. Serotonin and up-regulation of brain derived neurotropic factor (BDNF)
An intricate relationship seems to exist between serotonin and BDNF. Treatment with an SSRI can up-regulate BDNF levels (34,35), and BDNF can also stimulate serotonin synthesis (36).
BDNF is a critical component of synaptic maturation, synaptic plasticity, and neurogenesis (37–40). FXS can be classified as a disorder of the synapse (14,41,42). FMRP is highly expressed in neurons and plays an important role in dendritic plasticity (41,43–45). Without FMRP, dendrites do not develop normally. A hallmark morphological finding in patients with FXS is an abundance of immature dendritic spines (11,41,46–48). Dendritic-dependent changes involved in long-term depression and potentiation are impaired, contributing to the cognitive deficits seen in these patients. Given the synaptic abnormalities seen in patients with FXS, BDNF has been a focus of many FXS-related studies.
Serum levels of BDNF mRNA and BDNF protein are overall lower in patients with autism (49) and serum BDNF mRNA levels may positively correlate with IQ in patients with ASD (49). A crucial experiment by Lauterborn et al. (44) showed that impaired LTP (long term potentiation) in the Fmr1-KO mouse model is rescued when hippocampal slices are bathed in BDNF. This experiment evidenced that BDNF is affected by the absence of FMRP (47,50).
Though much attention is given to mature neurons, FMRP regulates proliferation and differentiation of adult neural stem/progenitor cells (51) and neurogenesis in early development (52). For example, FMRP is thought to play a crucial role in maintenance of radial glial cells (RGCs) in the neocortex during early development (53). Without FMRP, the RGC population is significantly reduced due to cell fate change from RGC to intermediate progenitor cell. In newborn neurons derived from neural progenitor cells lacking FMRP, basal levels of BDNF mRNA are increased (52,54). Levels of catalytic TrkB (tropomyosin-related kinase B), a receptor for BDNF, are also higher in the murine Fmr1 KO neural progenitor cells (54). This enhanced BDNF/TrkB signaling in FMRP-deficient progenitor cells likely contributes to the abnormal neural differentiation and migration patterns seen in the Fmr1 KO (43), such as the premature differentiation of neural progenitor cells which gives rise to neurons with small soma and short neurites (43). In addition, neural progenitor cells that lack FMRP also give rise to less glia (47).
However, the profile of BDNF expression appears to change significantly with age. In early mouse brain development, hippocampal expression of BDNF in the KO is still increased compared to WT (wild type) (54,55). However, by age 3–4 months, BDNF expression in the murine hippocampus is reduced compared to WT (52,55). Defects in hippocampal neurogenesis lead to cognitive deficits in the adult Fmr1 KO (56) and correlates with the hippocampal neurogenesis defects observed in individuals with FXS (57).
Experiments performed by Uutela et al. (55) provide mixed evidence as to whether BDNF is beneficial in the FXS mouse model. When Fmr1 KO mice were crossed with Bdnf+/− mice, the double transgenic mice showed roughly half of WT BDNF levels and deficits in water maze learning, contextual fear learning, and hippocampal neurogenesis. However, the double transgenic mice also showed improvements in locomotor activity, sensorimotor learning, and startle response in comparison to Fmr1 KO mice. Additionally, histological analysis of cultured neural progenitor cells showed that the double transgenic mice did not have the immature and abundant dendrites characteristically found in Fmr1 KO mice (55).
These mixed findings may be partially explained by considering the changing profile of BDNF levels during different stages of development. The double transgenic mice had relatively lower BDNF levels during early development when BDNF may be detrimentally overactive due to absence of normal reciprocal regulation by FMRP. In contrast, BDNF levels in the double transgenics are low in adulthood when its presence could be beneficial, as evidenced by Lauterborn et al. (44). It is unclear when in childhood BDNF stimulation would be beneficial and whether this is a critical mechanism for improvement with sertraline treatment.
5. SSRI treatment in FXS
Effective targeted treatments for FXS are being researched with a focus on mechanism-based approaches (58,59). These include agents targeting mGluR5, GABAA, the endocannabinoid system, and other signaling pathways such as insulin growth factor (IGF), MAPK/Erk, and BDNF (12,18,19,60–63). Symptom-based treatments currently include stimulants, antidepressants (e.g. selective serotonin reuptake inhibitors; SSRIs), and atypical antipsychotics which are useful in treating symptoms such as hyperactivity, anxiety, and aggression (59,64). SSRIs are sometimes prescribed for patients with FXS to relieve symptoms of anxiety (59). Anxiety is a classic feature in FXS throughout life and particularly in childhood (4,5). Recent evidence shows that SSRI treatment may confer non-classical benefits to patients with FXS as well (65). A core symptom in this patient population is difficulty in language acquisition and communication (1). Individuals may have abnormal speech rate, stuttering or exaggerated repetition, and a limited vocabulary. Oftentimes, patients fixate on a particular topic, word, or phrase and perseverate on these phrases or topics. Treatment with an SSRI may additionally benefit communication abilities in patients with FXS.
In 2011, Winarni et al. (65) performed a retrospective chart review of 45 children with FXS, aged 12–50 months. This analysis found that children with FXS who received the SSRI sertraline had significantly improved receptive and expressive language development compared to those not treated with sertraline. A subsequent controlled trial of sertraline in children with FXS ages 24 to 68 months is currently enrolling at the UC Davis MIND Institute (ClinicalTrials.gov identifier: NCT01474746) to assess the effects of sertraline in three general domains: early language/developmental abilities, sensory processing abilities, and symptoms relating to cognition, anxiety, and ASD.
6. Unique aspects of sertraline among the SSRIs
Clinical results and theoretical knowledge support the usefulness of SSRIs in treating patients with FXS (65). Sertraline may be relatively more effective than other SSRIs for this patient population. Sertraline has been approved by the Food and Drug Administration (FDA) as a treatment for OCD in children (age 6–17 years old) and main side effects are worsening of mood and/or behavior, irritability, aggression and suicidal thoughts. Other side effects include drowsiness, fatigue, dizziness, and sleep problems.
6.1. Dopamine reuptake inhibition
There is evidence that sertraline has unique neurochemical properties when compared to other SSRIs. Along with paroxetine, sertraline is considered one of the most potent inhibitors of serotonin reuptake (66). Additionally, sertraline significantly prevents dopamine reuptake (66). In a study done by Kitaichi et al. (67), researchers compared extracellular levels of serotonin, dopamine, and noradrenaline found in the prefrontal cortex, nucleus accumbens, and striatum of rats following administration of therapeutic doses of sertraline, fluvoxamine, or paroxetine. All agents successfully up-regulated serotonin in these areas, but sertraline was the only agent that also up-regulated dopamine, specifically in the nucleus accumbens and striatum.
Dopamine dysregulation is implicated in many neuropsychiatric conditions (66). Irregularities in dopamine production and/or dopamine receptors are linked to disorders such as autism, schizophrenia, depression, ADHD, and substance abuse. Abnormally high or low levels of dopamine negatively impact dendritic morphology (22,68,69). In a thorough review of the impact of dopamine on brain disorders and neurodevelopment, Money and Stanwood (68) state that examination of all the evidence points to dopamine playing “a crucial role…in formation and stabilization of synaptic connections in the striatum and frontal cortex”.
In vitro experiments done by Wang et al. (70) showed that dopamine receptor-mediated synaptic modulation is impaired in cells lacking FMRP. Normally, D1 stimulation leads to changes in AMPA receptor expression and phosphorylation necessary for LTP, both of which were significantly blunted in prefrontal cortical neurons derived from Fmr1 KO mice. This deficit was reversed when FMRP expression was induced in the cells via transfection. Furthermore, Wang et al. (70) showed that treating Fmr1−/− mice with a dopamine agonist specifically ameliorated the hyperactive behavior normally seen in these mice.
Further evidence of the importance of dopamine in FXS comes from the study discussed earlier by Lim et al. (23) that showed treatment of FXS hippocampal preparations with serotonin partially ameliorated in vitro LTP deficits by 20%. The researchers actually experimented further to discover that a particular low dose combination of a 5HT2B-R agonist and D1 receptor agonist restored GluA1-mediated LTP in hippocampal slices to 100% (wild-type levels). This finding was subsequently tested in vivo, yielding impressive results. Fmr1 KO mice were treated with either 5HT, the dopamine agonist, or both. The mice then underwent an associative learning task. Though there was mild improvement in each of the monotherapy groups, only the FXS mice receiving the combined 5HT and D1 cocktail were able to perform at WT levels, far superior to the abilities of their untreated FXS counterparts (23).
6.2. Neuroprotective effects
Taler et al. (35) analyzed in vitro cell (SHSY5Y human neuroblastoma cells) survival after 24 hours of antidepressant drug exposure including multiple SSRIs. Results showed that a low dose preparation (1–10 microgram) of sertraline or its derivative desmethylsertraline was the most beneficial SSRI in terms of cell survival. Compared to controls, sertraline improved cell survival rate by 50%. Paroxetine was the second most effective compound for cell survival, increasing viability by 40%. The rest of the drug candidates, which included fluoxetine, citalopram, reboxetine, venlafaxine, clomipramine, and mirtazapine showed no significant effects on cell survival. In a follow-up experiment, Taler et al. (35) compared the effects of sertraline on neuroblastoma cells exposed to stress (in the form of FCS-deprived media) vs. non-stressed conditions. The results showed that sertraline and desmethylsertraline administration during stress conditions increase cell survival, suggesting that sertraline has a neuroprotective effect (Figure 2).
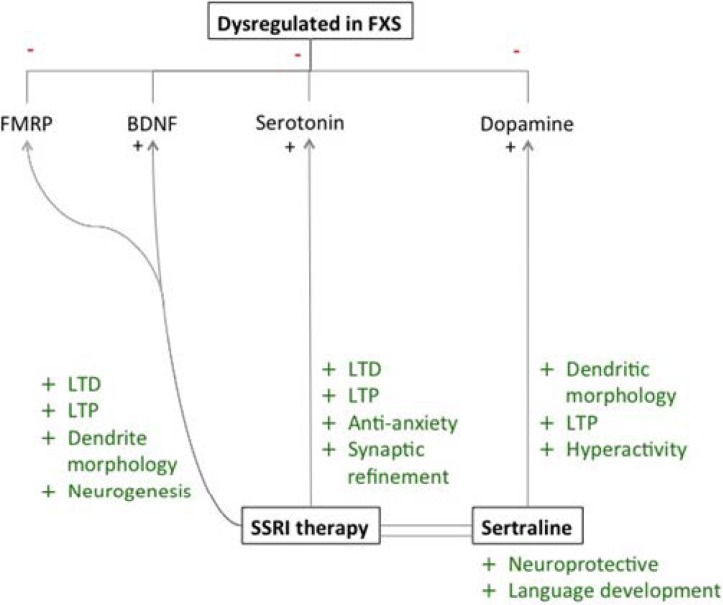
Figure 2.
Neurochemical effects of sertraline therapy in FXS. FMRP, BDNF, serotonin, and dopamine are all dysregulated in patients with FXS. Abnormal levels of FMRP and BDNF in FXS cause atypical dendritic morphology, LTD, LTP, and neurogenesis, all of which have been shown to normalize with serotonin application. Serotonin treatment may also directly benefit patients with FXS as an anxiolytic and by ameliorating defects in LTD, LTP and synaptic architecture. Sertraline may be an especially beneficial SSRI agent for FXS treatment because of its neurprotective effects and positive impact on language development. In addition, sertraline prevents reuptake of dopamine, another neurotransmitter thought to be dysregulated in FXS. Increasing dopamine levels in patients with FXS may help to improve hyperactivity and irregularities in LTP and dendritic morphology.
In subsequent in-vivo experiments by Taler et al. (35), four to six week old wild-type mice treated with 1mg/kg daily sertraline for 3 weeks showed improved performance on the Morris Water Maze (MWM) reacquisition phase. Older mice (12–14 months) performance on the reacquisition phase improved most when dosed at 10 mg/kg/day. Interestingly, no differences were observed in treated mice in the acquisition and extinction phases of the MWM. Compared to controls, sertraline-treated mice had increased BDNF expression in the hippocampus when dosed at 5 and 10 mg/kg/day. Additionally, phosphorylated ERK and Bcl-2 expression was up-regulated in young mice receiving 5 mg/kg/day, though not in older mice at any of the measured dosages. It is noteworthy that the more beneficial results occurred in the younger mice, lending more support to the theory that early intervention with sertraline may be more beneficial.
7. Conclusion
Serotonin enhances synaptic modulation and refinement (71). There is evidence that during the peak of synaptogenesis in brain development (birth to 5 years of life), there is a reduction of serotonin synthesis (28,32). In mice and humans, SSRIs can upregulate neurogenesis in the hippocampus. Pertinent to FXS, serotonin levels are likely affected by the lack of FMRP (24,28,33). Furthermore, other proteins that can be influenced by serotonin deficiency, such as BDNF, may contribute to the neurobiological deficits observed in FXS (34–36).
SSRIs are considered a symptomatic treatment for patients with FXS, but they may be working in a targeted manner as well. We have discussed evidence here that increasing serotonergic signaling can potentially rescue the neurobiology that is disrupted in FXS by upregulating levels of BDNF, increasing the number of GluA1 receptors and GlutA1-LTP, increasing levels of serotonin in the synapse, and by enhancing the dopaminergic system. These mechanisms are thought to improve synaptic plasticity and brain development. Other effects may include balancing cortical asymmetry of serotonin and overall neuroprotective effects.
Among the SSRIs, sertraline may be especially beneficial to patients with FXS due to its potency and ability to block the reuptake of dopamine, a neurotransmitter known to be dysregulated in FXS (71) and other neuropsychiatric conditions (68). Experiments done on murine FXS models show that treatment benefits vary depending on age (14). Serotonin and BDNF profiles change over time, and may be pathologically low in early development. Therefore, the timing of therapy with serotonergic agents may be extremely important in patients with FXS. Similarly, the consequences of FMRP expression depend on age (14). This is consistent with evidence from a retrospective chart review done by Winarni et al. (65), which found that one to four year old children with FXS who received sertraline showed improved receptive and expressive language outcomes. It is critical that this therapeutic opportunity is further investigated with controlled trials, as it could lead to significant improvements in symptoms, cognition, and quality of life for patients with FXS.
Acknowledgements
This work was supported by a grant from HRSA: R40MC22641, R40MC27701 and from the University of California Davis School of Medicine Student Research Fellowship Program.
References
- 1. Abbeduto L, Hagerman RJ. Language and communication in fragile X syndrome. Ment Retard Dev Disabil Res. 1997; 3:313-322. [Google Scholar]
- 2. Sudhalter V, Belser RC. Conversational characteristics of children with fragile X syndrome: Tangential language. Am J Ment Retard. 2001. September;106:389-400. [DOI] [PubMed] [Google Scholar]
- 3. Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Dev Neurosci. 2011; 33:379-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams TA, Langdon RM, Porter MA. Hyper-reactivity in fragile X syndrome females: Generalised or specific to socially-salient stimuli? A skin conductance study. Int J Psychophysiol. 2013; 88:26-34. [DOI] [PubMed] [Google Scholar]
- 5. Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. J Neurodev Disord. 2011; 3:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000; 38:1261-1270. [DOI] [PubMed] [Google Scholar]
- 7. Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. Am J Med Genet. 1999; 83:268-279. [PubMed] [Google Scholar]
- 8. Hagerman RJ, Hagerman PJ. “The Physical and Behavioral Phenotype.” Johns Hopkins University Press; 2002, Baltimore. [Google Scholar]
- 9. Bardoni B, Mandel JL, Fisch GS. FMR1 gene and fragile X syndrome. Am J Med Genet. 2000; 97:153-163. [DOI] [PubMed] [Google Scholar]
- 10. Tejada MI, Glover G, Martínez F, Guitart M, de Diego-Otero Y, Fernández-Carvajal I, Ramos FJ, Hernández-Chico C, Pintado E, Rosell J, Calvo MT, Ayuso C, Ramos-Arroyo MA, Maortua H, Milà M. Molecular testing for Fragile X: Analysis of 5062 tests from 1105 Fragile X Families - Performed in 12 clinical laboratories in Spain. BioMed Res Int. 2014; 2014:195793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: A twenty-year perspective. Annu Rev Pathol. 2012; 7:219-245. [DOI] [PubMed] [Google Scholar]
- 12. Hagerman RJ, Des-Portes V, Gasparini F, Jacquemont S, Gomez-Mancilla B. Translating molecular advances in fragile X syndrome into therapy: A review. J Clin Psychiatry. 2014; 75:e294-307. [DOI] [PubMed] [Google Scholar]
- 13. Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: Causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012; 122:4314-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zang T, Maksimova MA, Cowan CW, Bassel-Duby R, Olson EN, Huber KM. Postsynaptic FMRP bidirectionally regulates excitatory synapses as a function of developmental age and MEF2 activity. Mol Cell Neurosci. 2013; 56:39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gholizadeh S, Arsenault J, Xuan IC, Pacey LK, Hampson DR. Reduced phenotypic severity following adeno-associated virus-mediated Fmr1 gene delivery in fragile X mice. Neuropsychopharmacology. 2014: 39:3100-3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van der Molen MJ, Van der Molen MW. Reduced alpha and exaggerated theta power during the resting-state EEG in fragile X syndrome. Biol Psychol. 2013; 92:216-219. [DOI] [PubMed] [Google Scholar]
- 17. Lozano R, Hare EB, Hagerman RJ. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr Dis Treat. 2014; 10:1769-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hare EB, Hagerman RJ, Lozano R. Targeted treatments in fragile X syndrome. Expert Opin Orphan Drugs. 2014; 2:531-543. [Google Scholar]
- 19. Pop AS, Gomez-Mancilla B, Neri G, Willemsen R, Gasparini F. Fragile X syndrome: A preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development. Psychopharmacology. 2014; 231:1217-1226. [DOI] [PubMed] [Google Scholar]
- 20. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004; 27:370-377. [DOI] [PubMed] [Google Scholar]
- 21. Costa L, Spatuzza M, D'Antoni S, Bonaccorso CM, Trovato C, Musumeci SA, Leopoldo M, Lacivita E, Catania MV, Ciranna L. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of fragile X syndrome. Biol Psychiat. 2012; 72:924-933. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Hou L, Klann E, Nelson DL. Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. J Neurophysiol. 2009; 101:2572-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim CS, Hoang ET, Viar KE, Stornetta RL, Scott MM, Zhu JJ. Pharmacological rescue of Ras signaling, GluA1-dependent synaptic plasticity, and learning deficits in a fragile X model. Genes Dev. 2014; 28:273-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hessl D, Tassone F, Cordeiro L, Koldewyn K, McCormick C, Green C, Wegelin J, Yuhas J, Hagerman RJ. Brief report: Aggression and stereotypic behavior in males with fragile X syndrome - moderating secondary genes in a “single gene” disorder. J Autism Dev Disord. 2008; 38:184-189. [DOI] [PubMed] [Google Scholar]
- 25. McDuffie A, Kover ST, Hagerman RJ, Abbeduto L. Investigating word learning in fragile X syndrome: A fast-mapping study. J Autism Dev Disord. 2013, 43:1676-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iossifov I, Ronemus M, Levy D, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012; 74:285-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, Hagerman RJ. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008; 113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boccuto L, Chen CF, Pittman AR, Skinner CD, McCartney HJ, Jones K, Bochner BR, Stevenson RE, Schwartz CE. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism. 2013; 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDougle C, Naylor ST, Cohen DJ, Aghajanian GK, Heninger GR, Price LH. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiat. 1996; 53:993-1000. [DOI] [PubMed] [Google Scholar]
- 30. Kronk R, Bishop EE, Raspa M, Bickel JO, Mandel DA, Bailey DB., Jr Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep. 2010; 33:679-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, Goodlin-Jones BL. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009; 5:145-150. [PMC free article] [PubMed] [Google Scholar]
- 32. Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with α-[C-11] methyl-L-tryptophan. Synpase. 1998; 28:33-43. [DOI] [PubMed] [Google Scholar]
- 33. Chandana SR, Behen ME, Juhász C, Muzik O, Rothermel RD, Mangner TJ, Chakraborty PK, Chugani HT, Chugani DC. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005; 23:171-182. [DOI] [PubMed] [Google Scholar]
- 34. Bianchi P, Ciani E, Guidi S, Trazzi S, Felice D, Grossi G, Fernandez M, Giuliani A, Calzà L, Bartesaghi R. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci 2010; 30:8769-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taler M, Miron O, Gil-Ad I, Weizman A. Neuroprotective and procognitive effects of sertraline: In vitro and in vivo studies. Neuroscience Lett. 2013; 550:93-97. [DOI] [PubMed] [Google Scholar]
- 36. Altar CA, Boylan CB, Fritsche M, Jackson C, Hyman C, Lindsay RM. The neurotrophins NT-4/5 and BDNF augment serotonin, dopamine, and GABAergic systems during behaviorally effective infusions to the substantia nigra. Exp Neurol. 1994; 130:31-40. [DOI] [PubMed] [Google Scholar]
- 37. Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007; 134:4369-4380. [DOI] [PubMed] [Google Scholar]
- 38. Fukumitsu H, Ohtsuka M, Murai R, Nakamura H, Itoh K, Furukawa S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006; 26:13218-13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jansson LC, Louhivuori L, Wigren HK, Nordström T, Louhivuori V, Castrén ML, Åkerman KE. Brain-derived neurotrophic factor increases the motility of a particular Nmethyl-d-aspartate/GABA-responsive subset of neural progenitor cells. Neuroscience. 2012; 8:223-234. [DOI] [PubMed] [Google Scholar]
- 40. Alder J, Kramer BC, Hoskin C, Thakker-Varia S. Brain-derived neurotrophic factor produced by human umbilical tissue-derived cells is required for its effect on hippocampal dendritic differentiation. Dev Neurobiol. 2012; 72:755-765. [DOI] [PubMed] [Google Scholar]
- 41. Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010; 107:17768-17773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol 2012; 4:pii:a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castrén M, Tervonen T, Kärkkäinen V, Heinonen S, Castrén E, Larsson K, Bakker CE, Oostra BA, Åkerman K. Altered differentiation of neural stem cells in fragile X syndrome. Proc Natl Acad Sci U S A. 2005; 102:17834-17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lauterborn JC, Rex CS, Kramár E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007; 27:10685-10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003; 112:317-327. [DOI] [PubMed] [Google Scholar]
- 46. Bassell GJ, Warren ST. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron. 2008; 60:201-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Castrén ML, Castrén E. BDNF in fragile X syndrome. Neuropharmacology. 2014; 76:729-736. [DOI] [PubMed] [Google Scholar]
- 48. Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006; 26:7151-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taurines R, Segura M, Schecklmann M, et al. Altered peripheral BDNF mRNA expression and BDNF protein concentrations in blood of children and adolescents with autism spectrum disorder. J Neural Transm. 2014; 121:1117-1128. [DOI] [PubMed] [Google Scholar]
- 50. Castrén M, Lampinen KE, Miettinen R, Koponen E, Sipola I, Bakker CE, Oostra BA, Castrén E. BDNF regulates the expression of fragile X mental retardation protein mRNA in the hippocampus. Neurobiol Dis. 2002; 11:221-229. [DOI] [PubMed] [Google Scholar]
- 51. Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, Pfeiffer RL, Szulwach KE, Duan R, Barkho BZ, Li W, Liu C, Jin P, Zhao X. Fragile X mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010; 6:e1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Uutela M, Lindholm J, Rantamäki T, Umemori J, Hunter K, Võikar V, Castrén ML. Distinctive behavioral and cellular responses to fluoxetine in the mouse model for Fragile X syndrome. Front Cell Neurosci. 2014; 28:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saffary R, Xie Z. FMRP regulates the transition from radial glial cells to intermediate progenitor cells during neocortical development. J Neurosci. 2011; 31:1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Louhivuori V, Vicario A, Uutela M, Rantamäki T, Louhivuori LM, Castrén E, Tongiorgi E, Akerman KE, Castrén ML. BDNF and TrkB in neuronal differentiation of Fmr1-knockout mouse. Neurobiol Dis 2011; 41:469-480. [DOI] [PubMed] [Google Scholar]
- 55. Uutela M, Lindholm J, Louhivuori V, Wei H, Louhivuori LM, Pertovaara A, Akerman K, Castrén E, Castrén ML. Reduction of BDNF expression in Fmr1 knockout mice worsens cognitive deficits but improves hyperactivity and sensorimotor deficits. Genes Brain Behav. 2012; 11:513-523. [DOI] [PubMed] [Google Scholar]
- 56. Tervonen TA, Louhivuori V, Sun X, Hokkanen ME, Kratochwil CF, Zebryk P, Castrén E, Castrén ML. Aberrant differentiation of glutamatergic cells in neocortex of mouse model for fragile X syndrome. Neurobiol Dis. 2009; 33:250-259. [DOI] [PubMed] [Google Scholar]
- 57. Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra BA, Nelson DL, Jin P, Zhao X. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat Med. 2011; 17:559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Berry-Kravis E. Mechanism-Based Treatments in Neurodevelopmental Disorders: Fragile X Syndrome. Pediatr Neurol. 2014; 50:297-302. [DOI] [PubMed] [Google Scholar]
- 59. Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009; 123:378-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Busquets-Garcia A, Gomis-González M, Guegan T, Agustín-Pavón C, Pastor A, Mato S, Pérez-Samartín A, Matute C, de la Torre R, Dierssen M, Maldonado R, Ozaita A. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013; 19:603-607. [DOI] [PubMed] [Google Scholar]
- 61. Erickson CA, Wink LK, Ray B, Early MC, Stiegelmeyer E, Mathieu-Frasier L, Patrick V, Lahiri DK, McDougle CJ. Impact of acamprosate on behavior and brain-derived neurotrophic factor: An open-label study in youth with fragile X syndrome. Psychopharmacology (Berl). 2013; 228:75-84. [DOI] [PubMed] [Google Scholar]
- 62. Osterweil EK, Chuang SC, Chubykin AA, Sidorov M, Bianchi R, Wong RK, Bear MF. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013; 77:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Vries BB, Robinson H, Stolte-Dijkstra I, Tjon Pian Gi CV, Dijkstra PF, van Doorn J, Halley DJ, Oostra BA, Turner G, Niermeijer MF. General overgrowth in the fragile X syndrome: Variability in the phenotypic expression of the FMR1 gene mutation. J Med Genet. 1995; 32:764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome — present and future. Ment Retard Dev Disabil Rev. 2004; 10:42-48. [DOI] [PubMed] [Google Scholar]
- 65. Indah Winarni T, Chonchaiya W, Adams E, Au J, Mu Y, Rivera SM, Nguyen DV, Hagerman RJ. Sertraline may improve language developmental trajectory in young children with fragile X syndrome: A retrospective chart review. Autism Res Treat. 2012; 2012:104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders—I. Basic pharmacology. J Psychopharmacol. 1998; 12(3 Suppl B):5-20. [DOI] [PubMed] [Google Scholar]
- 67. Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, Koyama T. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharmacol. 2010; 647:90-96. [DOI] [PubMed] [Google Scholar]
- 68. Money KM, Stanwood GD. Developmental origins of brain disorders: Roles for dopamine. Front Cell Neurosci. 2013; 7:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol. 2009; 215:220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Ren M, Shang YZ, Xu H, Liu F, Zhao MG, Zhuo M. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008; 59:634-647. [DOI] [PubMed] [Google Scholar]
- 71. Krystal JH, Tolin DF, Sanacora G, Castner SA, Williams GV, Aikins DE, Hoffman RE, D'Souza DC. Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discov Today. 2009; 14(13–14):690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]