Abstract
Recently, the Q biotype of tobacco whitefly has been recognized as the most hazardous strain of Bemisia tabaci worldwide, because of its increased resistance to some insecticide groups. As an alternative control agent, we selected an Isaria javanica isolate as a candidate for the development of a mycopesticide against the Q biotype of sweet potato whitefly. To select optimal mass production media for solid-state fermentation, we compared the production yield and virulence of conidia between 2 substrates (barley and brown rice), and we also compared the effects of various additives on conidia production and virulence. Barley was a better substrate for conidia production, producing 3.43 × 1010 conidia/g, compared with 3.05 × 1010 conidia/g for brown rice. The addition of 2% CaCO3 + 2% CaSO4 to barley significantly increased conidia production. Addition of yeast extract, casein, or gluten also improved conidia production on barley. Gluten addition (3% and 1.32%) to brown rice improved conidia production by 14 and 6 times, respectively, relative to brown rice without additives. Conidia cultivated on barley produced a mortality rate of 62% in the sweet potato whitefly after 4-day treatment, compared with 53% for conidia cultivated on brown rice. The amendment of solid substrate cultivation with additives changed the virulence of the conidia produced; the median lethal time (LT50) was shorter for conidia produced on barley and brown rice with added yeast extract (1.32% and 3%, respectively), KNO3 (0.6% and 1%), or gluten (1.32% and 3%) compared with conidia produced on substrates without additives.
Keywords: Bemisia tabaci, Biological control, Entomopathogenic fungi, Isaria javanica, Mass production, Solid-state fermentation
The sweet potato whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is an important insect pest that can cause direct feeding damage and transmit plant pathogenic viruses. This species has shown fast development of resistance to some insecticide groups, especially in its Q biotype [1, 2, 3]. More than 600 plants, including tomato, pepper, beans, eggplant, cucumber, and other vegetables have been recorded worldwide as hosts of B. tabaci [4]. The Q biotype of B. tabaci has recently attracted international concerns because of its unusually rapid dispersal worldwide [2]. In Korea, biotype Q was collected in greenhouses cultivating cucumber, rose, sweet pepper, and tomato in 2005 [5], and pesticide-resistant populations were found in 2007 [6].
Entomopathogenic fungi are important components of the biological control of insect pests, and are especially widely employed against sucking type pests such as whitefly and aphids. Many fungal isolates have been reported as high pathogenicity fungi for the control of whitefly, for example: B. tabaci and Trialeurodes vaporariorum Westwood have been reported to be controlled by Beauveria bassiana (Bals.) Vuill., Aschersonia aleyrodis Webber, A. andropogonis Henn., A. goldiana Sacc. & Ellis, Isaria farinosa (Holmskiold) Fries (formerly Paecilomyces farinosus), I. fumosorosea Wize (formerly P. fumosoroseus (Wize) Brown & Smith), Isaria javanica (Friedrichs & Bally) Samson & Hywel-Jones, Lecanicillium muscarium (Petch) Zare and Gams (formerly Verticillium lecanii (Zimm.) Viegas), and Lecanicillium spp. (formerly V. lecanii) [7, 8, 9, 10, 11, 12, 13, 14]. Several of these entomopathogenic fungal isolates have been commercialized and widely applied in greenhouses or fields. For instance, the fungus B. bassiana (BotaniGard and Naturalis-O), and I. fumosorosea (PFR-97) are registered to control sweet potato whitefly in several countries including the USA, Colombia, Mexico, Brazil, Belgium, and Holland [8, 15]. An isolate of I. javanica (Garusajang) was recently enrolled as an environment-friendly organic agricultural material in Korea.
When fungal entomopathogens are sprayed onto crops to control foliar insect pests, a high dosage of conidia/spores is generally used [16]. Therefore, mass production of the spores of the infective fungi is important, especially for commercial use of the fungus. There are 3 systems generally used for the industrial scale production of entomopathogenic fungi: solid, liquid, and two-phase fermentation. Solid-state fermentation is the most common method used for the production of conidia, and this method uses grains such as barley, rice, and wheat as substrates [16]. Liquid fermentation is used for the production of blastospores. Increasing spore production yields is a major concern to researchers and commercial producers, since this is important for the practical application of fungi as mycopesticides. One obstacle to the use of fungal entomopathogens is a generally slow activity against target pests, and this factor is preventing the wider application and use of biocontrol agents. Therefore, the enhancement of virulence or triggering of infectivity during conidia production is important to improve the efficacy of entomopathogenic fungi for insect biocontrol.
Many studies have been conducted to simultaneously improve the spore production and virulence of entomopathogenic fungi by optimizing the cultivation conditions used for spore production. Media components may influence the spore yield, spore virulence, the activity of virulence-related enzymes such as Pr1, desiccation tolerance, and other relevant attributes of spores [17]. The addition of glucose, lecithin, collagen, or calcium chloride to liquid culture medium has been found to affect the production of entomopathogenic fungal spores [18, 19, 20]. For example, additives such as lecithin, collagen, or lactic acid into culture broth increased the production of Metarhizium anisopliae blastospores, but the virulence of the spores was weaker than that of spores produced on media without additive [19]. The addition of casamino acid into culture broth increased the production yield of M. anisopliae spores and improved their virulence against Aedes aegypti [21]. Addition of yeast extract and peptone into liquid media improved both the production of Beauveria brongniartii spores and their virulence against T. vaporariorum [22]. The yield of B. brongniartii spores was found to vary depending on the combination and doses of additives used for their production, with the additives investigated including calcium chloride, chitin, polyethylene glycol, lactic acid, lecithin, molasses, and nutrient media [20]. Conidia of B. bassiana and M. anisopliae produced using different medium compositions or osmotic stress medium showed differences in their germination rate, Pr1 activity, and virulence [23, 24]. Most of these studies were mainly conducted using semi-synthetic or synthetic media for the production of blastospores or conidia. To date, few studies have investigated methods to improve conidia production and virulence on solid substrates such as grains.
Most of the methods used for the large-scale production of conidia of fungal entomopathogens involve the use of grains or other cheap materials. Therefore, this study was conducted to investigate the mass production of conidia of I. javanica, comparing between barley and brown rice as substrates and also comparing among the use of different additives for their effects on conidia yield and the virulence of conidia against sweet potato whitefly. The addition of gluten to brown rice improved conidia production and virulence, and conidia yield on barley was increased by the addition of CaCO3 and CaSO4.
MATERIALS AND METHODS
An I. javanica isolate, which was isolated from a fungus-infected Aleyrodidae adult in Korea and showed high pathogenicity against the B. tabaci biotype Q [14], was kept in 10% (v/v) glycerol at -20℃. The isolate was initially cultivated on potato dextrose agar (PDA) at 25 ± 1℃ for 14 days. For the mass production of this I. javanica isolate, brown rice and barley were purchased and stored in a refrigerator at 4℃ until use.
To use grains as a substrate for conidia production, the 2 grains were soaked in tap water for 2 hr and placed into a plastic basket for 1 hr to remove water. One hundred grams of grain was put into an autoclavable plastic bag and autoclaved at 121℃ for 60 min. To understand the effects of different additives on conidia production by solid-state cultivation using grains as a substrate, a carbon or nitrogen source or trace minerals were added into 15mL conidia suspension (1 × 105 conidia/mL) and dissolved. The additives investigated were as follows: 2% (w/v) soluble starch (Daejung Co., Busan, Korea), 2% (w/v) mannose (Sigma-Aldrich Co., St. Louis, MO, USA), 2% (w/v) CaCO3 and 2% (w/v) CaSO4 (Sigma-Aldrich Co.), 1.32% and 3% (w/v) of hydrolyzed casein (Sigma-Aldrich Co.), 1.32% and 3% (w/v) of Difco yeast extract (Becton Dickinson Co., Franklin Lakes, NJ, USA), 0.6% and 1% (w/v) of KNO3 (Sigma-Aldrich Co.), and 1.32% and 3% (w/v) of hydrolyzed wheat gluten protein (Sigma-Aldrich Co.). The conidia suspension was then inoculated onto barley or brown rice in a plastic bag, mixed well, and incubated at 25 ± 1℃ for 15 days. Three grams of barley or brown rice was randomly taken from each culture. The grain was added to 27 mL 0.05% sterilized Tween 80, vortexed for 1 min, and then the suspension was filtered through 4 layers of sterilized cheesecloth. The number of conidia was counted using an improved Neubauer haemocytometer (Sigma-Aldrich Co.). In each trial, each cultivation condition was replicated in 3 different bags, and the trial was repeated 4 times.
To test the virulence of conidia produced using solid substrates and different additives, each conidia suspension was sprayed onto second instar nymphs of tobacco whitefly, as previously described [14]. Briefly, to prepare synchronized nymphs, fifteen pairs of adult whiteflies were infested overnight on a potted eggplant seedling with 1 leaf, which was prepared by removing the other leaves from a 30-day-old pruned whole eggplant seedling, and then the adult whiteflies were removed. The seedlings were grown in a Plexiglas cage (35 × 40 × 40 cm) at a constant temperature of 25 ± 1℃ with a 16L : 8D lighting schedule for 11 days until the whiteflies reached their second instar.
For virulence tests, conidia were harvested as described above from the barley or brown rice cultures amended with different additives after 15 days cultivation. The eggplant leaf discs infested with second instar nymphs of B. tabaci were sprayed with each conidia suspension at 3 different concentrations (105, 106, and 107 conidia/mL). One milliliter of each suspension was applied to leaf discs using a spray box (90 × 90 × 90 cm) fitted with a polyvinyl acetal cone nozzle (Φ 1.5 mm). The spray tower was a Plexiglas cylinder and the spray nozzle was installed through the top layer and connected to a vacuum pump, which was fixed at the pressure setting of 100 kPA. The number of conidia applied to each leaf disc was estimated by counting the spores that landed on a Petri dish (Φ 3.5 cm) containing 2 mL of 0.05% Tween 80 that was placed beside the eggplant seedlings during the spore application. The total number of conidia sprayed within the Petri dish area was determined using a hemocytometer. The average conidia deposition on each leaf disc was calculated to be 2.8~3.2 conidia/mm2 for the application of 106 conidia/mL and 10.7~13.6 conidia/mm2 for the application of 107 conidia/mL. Spore viability was determined by germination tests of the conidia suspensions after 24 hr incubation at 25 ± 1℃ on PDA medium. Spore viabilities were 99.2 ± 0.4%. This bioassay was conducted twice. Three replicate dishes were investigated per treatment in each trial.
One-way analysis of variance [25] was used to compare conidia production and mortality among the different additives used for cultivation on each grain substrate. Each separate trial was considered as a block. Median lethal time (LT50) was estimated using the LIFEREG procedure and data were fitted to a Weibull distribution [25]. The differences in conidia yield, mortality and LT50 at 2 different time points (4 and 6 days after treatment) among different additives in each grain were compared using the least significant difference test (α < 0.05).
RESULTS
The production of I. javanica conidia differed among the substrate and additive combinations investigated after 15 days cultivation (brown rice, F = 8.48; df = 11, 47; p < 0.0001 and barley, F = 3.78; df = 11, 47; p = 0.0012). Barley (3.43 × 1010 conidia/g) was a higher-yielding solid substrate for conidia production than brown rice (3.05 × 1010 conidia/g). On barley, the addition of 2% CaCO3 and 2% CaSO4 significantly increased conidia production. The addition of yeast extract, casein, or gluten to barley improved conidia production compared with cultivation on barley without additives. When starch or mannose was mixed with barley as the solid substrate, the number of conidia produced was lower (2.33 × 1010 and 2.15 × 1010 conidia/g, respectively) than the number produced by cultivation without additives (3.43 × 1010 conidia/g).
The addition of gluten (3% and 1.32%) to brown rice improved the conidia production yield by 14 and 6 times, respectively, compared with that of brown rice without additives (3.05 × 1010 conidia/g). Starch addition to brown rice increased conidia production, in contrast with barley. The addition of potassium nitrate (KNO3), yeast extract, 2% CaCO3 and 2% CaSO4, or mannose to brown rice did not increase conidia production compared with cultivation on brown rice without additives. The addition of gluten stimulated conidia production on both solid substrates, but the addition of mannose and KNO3 suppressed the production of conidia of the I. javanica isolate following cultivation on barley and brown rice.
Conidia virulence differed between the solid substrates (Table 1). The mortality of sweet potato whitefly treated with the conidia suspension produced on barley was generally higher (13% at 105 conidia/mL, 36% at 106 conidia/mL, and 62% at 107 conidia/mL) than that of whitefly treated with the conidia suspension produced on brown rice (14%, 23%, and 53%, respectively) at 4 days after treatment (pooled t tests: 105 conidia/mL, t = 0.16, p = 0.8880; 106 conidia/mL, t = -0.55, p = 0.6374; 107 conidia/mL, t = -0.84, p = 0.4889). However, these changes were not significant, and conidia virulence showed non-significant trends toward differences between the solid substrates. Differences in mortality at the sixth day after application showed trends similar to those on the fourth day, with mortality rates of 57%, 81%, and 100% for barley-cultivated conidia and 46%, 74%, and 100% for brown rice at the 3 concentrations of conidia applied, respectively.
Table 1.
Mortality of second instar nymphs of sweet potato whitefly at the fourth and sixth days after treatment with suspensions of Isaria javanica conidia produced by solid state cultivation on brown rice and barley without additives
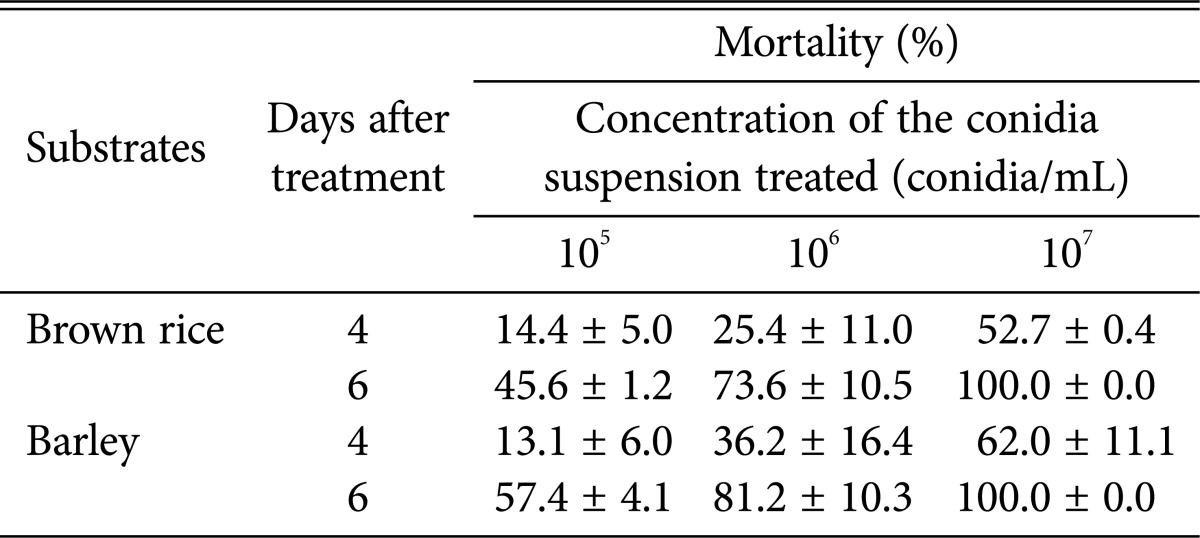
Values are presented as mean ± SE.
The inclusion of additives in the solid-state cultivation procedure changed conidia virulence (Fig. 1), which varied among the different additive and substrate combinations investigated. Conidia produced on barley with different additives did not statistically increase whitefly mortality at 105, 106, and 107 conidia/mL treatment (ANOVA: 105 conidia/mL, F = 0.41, df = 11, 23, p = 0.9236; 106 conidia/mL, F = 1.04, df = 11, 23, p = 0.4681; 107 conidia/mL, F = 4.19, df = 11, 23, p = 0.0103). The median lethal time (LT50) was shorter for conidia produced with the addition of mannose and starch at 105 (F = 0.58; df = 11, 23; p = 0.8096), 3% gluten and 1% KNO3 at 106 (F = 0.41; df = 11, 23; p = 0.9228), and 1.32% and 3% yeast extract, 0.6% and 1% KNO3, and 1.32% and 3% gluten at 107 conidia/mL treatment (F = 30.80; df = 11, 23; p < 0.0001).
Fig. 1.
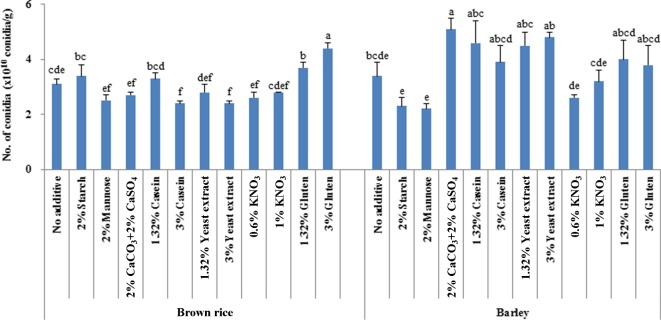
Conidia production of Isaria javanica on different substrates amended with additives for 15 days at 25 ± 1℃. Different letters at the each grain (brown rice and barley) are significantly different (p < 0.05, least significant difference test).
Mortality of whitefly treated with conidia that were cultivated on brown rice with additives was lower than that of the conidia produced on brown rice without additives although this difference was not significant (Table 2). The LT50 differed among additives; at 105 conidia/mL, the addition of 1.32% gluten and 1.32% casein resulted in lower LT50 values (6.2 days for both additives, compared with 6.3 days for substrate without additives) (F = 1.00; df = 11, 23; p = 0.4962), while at 106 conidia/mL, the addition of 0.6% and 1% KNO3, 1.32% gluten, and 1.32% yeast extract resulted in LT50 values of 4.6 days, 4.4 days, 4.5 days, and 4.6 days, respectively, compared with 4.7 days for conidia produced by cultivation without additives (F = 0.50; df = 11, 23; p = 0.8716), and at 107 conidia/mL, the addition of 1.32% and 3% gluten, 0.6% and 1% KNO3 and 3% yeast extract led to LT50 values of 3.1 days, 3.3 days, 3.5 days, 3.4 days, and 3.5 days, which were significantly decreased compared with the LT50 of 3.8 days for conidia produced by cultivation without additives (F = 11.73; df = 11, 23; p < 0.0001).
Table 2.
Median lethal time (LT50) of second instar nymphs of the sweet potato whitefly following treatment with suspensions of Isaria javanica conidia produced by solid state fermentation on brown rice and barley with comparison between different additives
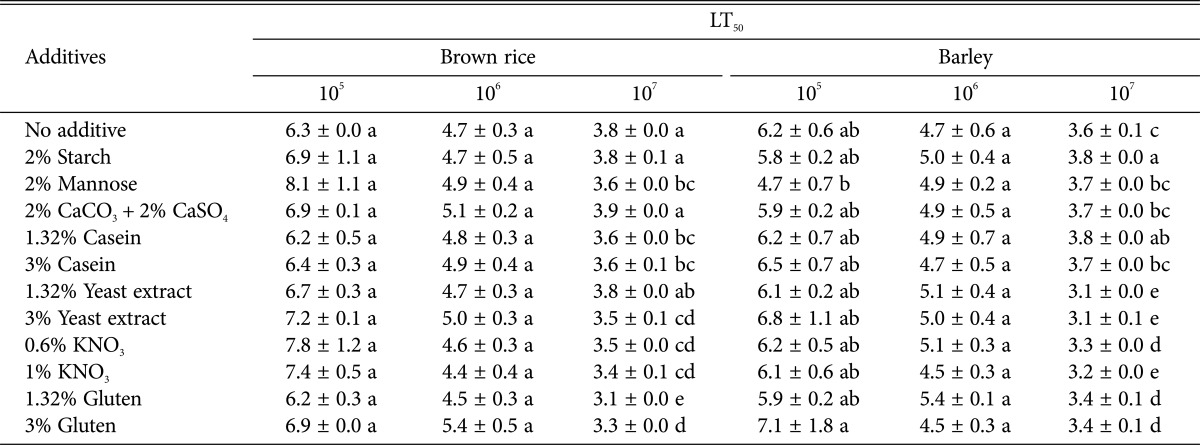
Different letters in the same column indicate significant differences (p < 0.05, least significant difference test).
DISCUSSION
The production yield and virulence of I. javanica conidia varied among different combinations of additives and solid substrates used for cultivation. Gluten and KNO3 addition commonly increased the conidia production and virulence of the Korean isolate of I. javanica under the different conditions investigated.
A P. fumosoroseus isolate produced more blastospores in liquid medium that was supplemented with casein or yeast extract than in unsupplemented medium [26]. Similarly, tobacco whitefly pathogenic P. fumosoroseus produced more blastospores in liquid culture media supplemented with glucose and casamino acid [27]. The addition of casein, yeast extract, or corn gluten as nitrogen sources, and of glucose or casein as carbon sources into liquid culture media increased the production yield of P. fumosoroseus spores, and the spores also showed enhanced tolerance to dessication [28]. Casein and yeast extract supplementation commonly increased the production of P. fumosoroseus blastospores in different liquid media. While many studies have previously investigated the effects of different liquid media and additives on the production and virulence of entomopathogenic fungal spores, few studies have previously been conducted on solid media.
P. fumosoroseus, which was isolated from the cadavers of lepidopteran caterpillars in India, showed higher conidia production when cultivated on sorghum (10.4 × 1011 spores/100 g) than when cultivated on corn, rice, pearl millet, or wheat [29]. Sporulation of M. anisopliae was stimulated on agar medium by the addition of starch, mannose, or KNO3 [30]. Soluble starch and mannose were the most effective stimulators of M. anisopliae sporulation. In this study, when the I. javanica isolate was cultivated on brown rice or barley with the addition of starch or mannose, conidia production was decreased. Conidia of M. anisopliae cultured in Sabouraud dextrose agar with added KCl showed a decreased germination rate and the strength of spore attachment onto the host cuticle differed from that of conidia produced in liquid culture media [31]. Nomuraea rileyi produced more conidia when cultivated on wheat with added yeast extract than when cultivated on wheat with added brewer's yeast or whole milk powder [32]. Rice was the best solid substrate for the production of V. lecanii spores, compared with sorghum, corn, ragi, and wheat. The sporulation of P. lilacinus, which was isolated from nematodes, was stimulated when it was cultivated on agar medium containing added mannose, sucrose, or starch. Different P. lilacinus isolates showed varying nutrient requirements for sporulation [33]. The addition of 2% CaCO3 and 2% CaSO4 into grain cultivation bags prevented grains from sticking together and thus provided an increased surface area for fungal growth [34]. In our study, the I. javanica isolate showed good sporulation on barley. When the isolate was cultivated on barley with added CaCO3 plus CaSO4 or gluten, conidia production was significantly increased, but mannose, starch, or KNO3 addition inhibited conidia production. Thus, the use of additives for the solid phase cultivation of entomopathogenic fungi led to differences in the yields of conidia production. For the mass production of fungal conidia, each isolate needs to be studied to determine the optimal solid substrate and additive ingredient(s).
Spore attachment, germination, and the activity of enzymes, including proteases such as Pr1, are important factors for fungal virulence. These characteristics may be influenced by spore production conditions such as nutritional ingredients in the cultivation media. The influence of additives such as casamino acid on the spore production and virulence of various fungal isolates has been reported to vary among different media. For example, a M. anisopliae isolate cultured in medium with added casamino acids showed increased 40% mortality against A. aegypti compared with the same isolate cultured in medium without added casamino acids [21]. According to Shah et al. [24], M. anisopliae produced highly virulent conidia when cultivated in osmotic stress medium, and the conidia showed higher Pr1 enzyme activity. The addition of yeast extract and peptone to cultivation media also increased the Pr1 activity of M. anisopliae conidia, but the relationship between virulence and Pr1 activity was not clear in that study [23]. The addition of lecithin, collagen, or lactic acid into culture broth increased the production of M. anisopliae blastospores, but the virulence of the spores was similar or lower than that of spores produced in media without additives [19]. When conidia of Lecanicillium sp. were produced by cultivation in media with the addition of 2% glucose and sprayed at low concentration (106 conidia/mL), the infection rate of whitefly was higher than that of conidia produced by cultivation in media with the addition of maltose and peptone. However, at a higher dosage (108 conidia/mL), there was no significant difference between the infection rates of the conidia produced by cultivation with different additives [22]. The addition of trace minerals such as KJ, ZnSO4, or KBr to agar medium increased the pathogenicity of B. bassiana conidia [35]. For I. javanica, which is a sweet potato whitefly pathogen, the virulence of conidia was increased when the isolate was cultured with the addition of gluten on both brown rice and barley solid substrates. Conidia cultivated on barley with added KNO3 or yeast extract also showed improved mortality against tobacco whitefly, compared with conidia cultivated on barley without additives. We expected that when the isolate was cultured with additives, highly virulent conidia would be produced, allowing us to spray a low concentration of spores onto crops but still achieve a high control efficacy against whitefly. However, against our expectations, the virulence of the conidia was only improved at a high spraying concentration (107 conidia/mL). At spraying concentrations of 105 and 106 conidia/mL, the LT50 did not significantly differ among the conidia produced by cultivation on solid substrates with different additives. These results were different from those of Shi et al. [22], whose study found that the addition of glucose to cultivation media led to the production of V. lecanii conidia that produced a higher mortality rate in their host (T. vaporariorum). We will conduct future studies to investigate why virulence was increased by the addition of gluten, KNO3, or yeast extract at the highest spraying concentration of conidia. In conclusion, the production of sweet potato whitefly pathogenic I. javanica conidia was increased on barley by the addition of CaCO3+CaSO4 and on brown rice by the addition of gluten. Gluten addition also improved the virulence of conidia cultivated on both substrates. Compared with conidia produced on barley without additives, conidia produced on barley with added KNO3 or yeast extract also showed improved virulence against tobacco whitefly.
ACKNOWLEDGEMENTS
This study was supported by research grants (PJ010049022014) provided by the Rural Development Administration (RDA) of Korea.
References
- 1.De Barro PJ, Liu SS, Boykin LM, Dinsdale AB. Bemisia tabaci: a statement of species status. Annu Rev Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- 2.Scott IA, Workman PJ, Drayton GM, Burnip GM. First record of Bemisia tabaci biotype Q in New Zealand. N Z Plant Prot. 2007;60:264–270. [Google Scholar]
- 3.Jones DR. Plant viruses transmitted by whiteflies. Eur J Plant Pathol. 2003;109:195–219. [Google Scholar]
- 4.Oliveira MR, Henneberry TJ, Anderson P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001;20:709–723. [Google Scholar]
- 5.Lee M, Kang S, Lee S, Lee HS, Choi JY, Lee GS, Kim WY, Lee SW, Kim SG, Uhm KB. Occurrence of the B- and Qbiotypes of Bemisia tabaci in Korea. Korean J Appl Entomol. 2005;44:169–175. [Google Scholar]
- 6.Kim EH, Sung JW, Yang JO, Ahn HG, Yoon C, Seo MJ, Kim GH. Comparison of insecticide susceptibility and enzyme activities of biotype B and Q of Bemisia tabaci. Korean J Pestic Sci. 2007;11:320–330. [Google Scholar]
- 7.Wraight SP, Carruthers RI, Jaronski ST, Bradley CA, Garza CJ, Galaini-Wraight S. Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Control. 2000;17:203–217. [Google Scholar]
- 8.Faria M, Wraight SP. Biological control of Bemisia tabaci with fungi. Crop Prot. 2001;20:767–778. [Google Scholar]
- 9.Al-Deghairi MA. Bioassay evaluation of the entomopathogenic fungi, Beauveria bassiana Vuellemin against eggs and nymphs of Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) Pak J Biol Sci. 2008;11:1551–1560. doi: 10.3923/pjbs.2008.1551.1560. [DOI] [PubMed] [Google Scholar]
- 10.Cabanillas HE, Jones WA. Pathogenicity of Isaria sp. (Hypocreales: Clavicipitaceae) against the sweet potato whitefly B biotype, Bemisia tabaci (Hemiptera: Aleyrodidae) Crop Prot. 2009;28:333–337. [Google Scholar]
- 11.Saito T, Sugiyama K. Pathogenicity of three Japanese strains of entomopathogenic fungi against the silverleaf whitefly, Bemisia argentifolii. Appl Entomol Zool. 2005;40:169–172. [Google Scholar]
- 12.Cuthbertson AG, Walters KF. Pathogenicity of the entomopathogenic fungus, Lecanicillium muscarium, against the sweetpotato whitefly Bemisia tabaci under laboratory and glasshouse conditions. Mycopathologia. 2005;160:315–319. doi: 10.1007/s11046-005-0122-2. [DOI] [PubMed] [Google Scholar]
- 13.Meekes ET, Fransen JJ, van Lenteren JC. Pathogenicity of Aschersonia spp. against whiteflies Bemisia argentifolii and Trialeurodes vaporariorum. J Invertebr Pathol. 2002;81:1–11. doi: 10.1016/s0022-2011(02)00150-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Kim JJ. Susceptibility of the tabacco whitfly, Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q to entomopathogenic fungi. Biocontrol Sci Technol. 2011;21:1471–1483. [Google Scholar]
- 15.de Faria MR, Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43:237–256. [Google Scholar]
- 16.Jackson MA, Dunlap CA, Jaronski ST. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl. 2010;55:129–145. [Google Scholar]
- 17.Qiu Z, Song F, Qiu Y, Li X, Guan X. Optimization of the medium composition of a biphasic production system for mycelial growth and spore production of Aschersonia placenta using response surface methodology. J Invertebr Pathol. 2013;112:108–115. doi: 10.1016/j.jip.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Vega FE, Jackson MA, Mercadier G, Poprawski TJ. The impact of nutrition on spore yields for various fungal entomopathogens in liquid culture. World J Microbiol Biotechnol. 2003;19:363–368. [Google Scholar]
- 19.Kleespies RG, Zimmermann G. Effect of additives on the production, viability and virulence of blastospores of Metarhizium anisopliae. Biocontrol Sci Technol. 1998;8:207–214. [Google Scholar]
- 20.Srikanth J, Santhalakshmi G. Effect of media additives on the production of Beauveria brongniartii, an entomopathogenic fungus of Holotrichia serrata. Sugar Tech. 2012;14:284–290. [Google Scholar]
- 21.Maldonado-Blanco MG, Gallegos-Sandoval JL, Fernández-Peña G, Sandoval-Coronado CF, Elías-Santos M. Effect of culture medium on the production and virulence of submerged spores of Metarhizium anisopliae and Beauveria bassiana against larvae and adults of Aedes aegypti (Diptera: Culicidae) Biocontrol Sci Technol. 2014;24:180–189. [Google Scholar]
- 22.Shi Z, Li M, Zhang L. Effects of nutrients on germination of Verticillium lecanii (= Lecanicillium sp.) conidia and infection of greenhouse whitefly, Trialeurodes vaporariorum. Biocontrol Sci Technol. 2006;16:599–606. [Google Scholar]
- 23.Safavi SA, Shah FA, Pakdel AK, Reza Rasoulian G, Bandari AR, Butt TM. Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol Lett. 2007;270:116–123. doi: 10.1111/j.1574-6968.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- 24.Shah FA, Wang CS, Butt TM. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett. 2005;251:259–266. doi: 10.1016/j.femsle.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute Inc. SAS OnlineDoc® 9.1.3. Cary: SAS Institute Inc.; 2012. [Google Scholar]
- 26.Jackson MA, Cliquet S, Iten LB. Media and fermentation processes for the rapid production of high concentrations of stable blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. Biocontrol Sci Technol. 2003;13:23–33. [Google Scholar]
- 27.Jackson MA, McGuire MR, Lacey LA, Wraight SP. Liquid culture production of desiccation tolerant blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. Mycol Res. 1997;101:35–41. [Google Scholar]
- 28.Jackson MA. Method for producing desiccation tolerant Paecilomyces fumosoroseus spores. U.S. Patent No. 5968808. Beltsville: Department of Agriculture, United States of America; 1997. [Google Scholar]
- 29.Sahayaraj K, Namasivayam SK. Mass production of entomopathogenic fungi using agricultural products and by products. Afr J Biotechnol. 2008;7:1907–1910. [Google Scholar]
- 30.Li DP, Holdom DG. Effects of nutrients on colony formation, growth, and sporulation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) J Invertebr Pathol. 1995;65:253–260. [Google Scholar]
- 31.Ibrahim L, Butt TM, Jenkinson P. Effect of artificial culture media on germination, growth, virulence and surface properties of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycol Res. 2002;106:705–715. [Google Scholar]
- 32.Holdom DG, van de Klashorst G. Inexpensive culture media and methods for Nomuraea rileyi. J Invertebr Pathol. 1986;48:246–248. [Google Scholar]
- 33.Sun M, Liu X. Carbon requirements of some nematophagous, entomopathogenic and mycoparasitic hyphomycetes as fungal biocontrol agents. Mycopathologia. 2006;161:295–305. doi: 10.1007/s11046-006-0249-9. [DOI] [PubMed] [Google Scholar]
- 34.Derakhshan A, Rabindra RJ, Ramanujam B, Rahimi M. Evaluation of different media and methods of cultivation on the production and viability of entomopathogenic fungi, Verticillium lecanii (Zimm.) Viegas. Pak J Biol Sci. 2008;11:1506–1509. doi: 10.3923/pjbs.2008.1506.1509. [DOI] [PubMed] [Google Scholar]
- 35.Iskandarov US, Guzalova AG, Davranov KD. Effects of nutrient medium composition and temperature on the germination of conidia and the entomopathogenic activity of the fungi Beauveria bassiana and Metarhizium anisopliae. Appl Biochem Microbiol. 2006;42:72–76. [PubMed] [Google Scholar]


