Abstract
Background:
Medicinal herbs such as Citrullus Colocynthis (C.C) have been used traditionally in the treatment of diabetes mellitus. However therapeutic applications and adverse effects of C.C and its natural variants are not determined well. The current work investigates the effects of pulp and seed extract of C.C on hepatocyte's glycogen stores.
Materials and Methods:
Thirty six male rabbits were divided into six groups (control and diabetic) randomly. Alloxan was used in order to induce diabetes mellitus in animals. Among 5 diabetic groups, one remained as control and the rest received 100 and 200 mg/kg/day of either pulp or seed extract. One month later, animals were sacrificed and their liver specimen fixed in 10% Formalin was stained with periodic acid schiff (PAS) for light microscopic scanning.
Results:
PAS staining of hepatocytes revealed large amounts of glycogen stores in diabetic animals treated with pulp and seed extracts of C.C, contrary with non-treated diabetic rabbits. Sites of glycogen deposition were also different in animals treated with seed extract (P < 0.0001). No hepatic congestion was seen in treated animals. Dose escalation has no effect on the obtained results.
Conclusions:
The anti-diabetic effects of C.C can be explained by its effects on accumulation of glycogen stores in hepatocytes. The importance of varied sites of glycogen deposition by the application of C.C needs to be determined.
Keywords: Citrullus Colocynthis, diabetes mellitus, glycogen stores
INTRODUCTION
Type II diabetes mellitus (T2DM) is a common disease with substantial morbidity and mortality.[1] Current available medications for T2DM as life style modification, oral hypoglycemic agents, and insulin are with their own limitations and side effects.[2] Alternative medications as natural products and herbal medicine with experimental and clinical antidaibetic activity seem to be of great value in this regard. Traditional medicinal herbs with their extensive resources are natural products without obvious toxic side effects.[3] The antidaibetic activity of natural products is attributed to the terpenoids, alkaloids, phenolics and some other ingredients.[2] By now, the only approved antidaibetic drug derived from herbal sources with the long history of use for diabetes is the biguanide Metformin from French lilac (Galega officinalis).[4] But there are emerging clinical data which support antidaibetic indications for several other herbs.[4]
Citrullus colocynthis (colocynth) (C.C) is a member of the Cucurbitaceae superfamily, which is used as a traditional good remedy for the DM.[5] This plant is also known as Hindal in Arabian word[6] and Abu Jahl watermelon in Persian speaking. Due to its bitter flavor, similar to colocynthine, the extract of this fruit was named so. Its ingredients are glycosides (Estrols), alkaloids and flavinoids.[7,8] Estrols (Class A, B, K, L, J and E) are other constituents of C.C.[9,10]
The curcurbitacins (highly oxygenated tetracycluic glycosides) are of great interest for therapeutic applications due to their wide board of biological functions exert in plants and animals.[11] These compounds are predominantly found in curcucbitaceae family but are also present in other families of plant kingdom.[11] Another member of this family, Mormordica charantia is a popular traditional remedy for DM. C.C is also applicable as purgative, antineoplastic, anti-allergic and anti-rheumatism.[12,13]
The dried pulp and seed extracts of C. C are both considered as anti-diabetic agents.[14,15] However, the fruit is mostly used in its intact form traditionally. C.C has been shown to decrease plasma glucose levels in both normo- and hyperglycemic cases.[7] Despite of these known effects, the underlying mechanisms and effective ingredients are not yet clear. The current study aims to investigate the impact of pulp and seed of C.C on the hepatic glycogen stores of pharmacologically diabetic rabbits.
MATERIALS AND METHODS
Extraction
The full process was done by a pharmacologist, in Dept. of Pharmacology, Tabriz University of Medical Sciences, Tabriz, Iran. Seeds of dried C.C were separated from pulp, rind and milled mechanically in order to obtain fine particles. Derived powder was then defatted by n-hexan. Extraction process from pulp and defatted seed was done via application of 70% methanol and repeated for three times. The extract was dried at low temperature (<40°C) and under pressure less than 100 mmHg in a rotator evaporator. The dried extract was stored in zero temperature till application, which was reconstituted in 1 ml of distilled water to prepare either 10% or 20% solutions. In all experiments, the same amount was applied.
Phytochemical analysis
Estrols investigation: Estrols were identified by the creation of blue-green color after addition to anidric acetic plus concentrated sulphoric acid, based on the Libermann-Burchard reaction.
Flavonoids investigation: Using Cyanidin test, Flavonoids were detected by production of red or orange color by addition of Hydrochloric acid (HCL) and Mg ribbon in alcoholic milieu.
Saponin investigation: Aqueous extracts containing saponin form permanent foam after 2 minutes of vigorous shaking at room temperature. Olive addition in the main solution, leads in emulsion formation.
Alkaloid investigation: Under the presence of Mayer or Dragendorff alkaloid sin acidic solution (1% HCL) get turbid and deposit at the bottom of test tube.[16]
Animals
The present study was conducted using 3 month old New Zealand white male rabbits, weighing approximately 3 kg. Thirty six rabbits were randomly assigned into 6 groups randomly (each consisting of 6 rabbits): One group was normal control which received regular rabbit chow for 4 weeks (normal group) and five groups as test group, in which diabetes was induced in them. Animals were fed by regular diet with free access to water. Animals in test group, similar to the control rabbits, received the regular diet with free access to tap water. In order to induce diabetes in test animals, single dose of Alloxan (100 mg/kg body wt, Sigma, USA) was injected intraveneously. One week later, the induction of diabetes was confirmed by the appearance of hyperglycemic state in treated animals. Among diabetic animals, one group remained as control and the other received orally 100 and 200 mg/kg/day for 4 weeks, of either pulp (2 groups) or seed extract of C.C (2 groups). Control rabbits had the same conditions as diabetic ones rather than receiving the above mentioned treatment. All treated animals were alive and no side effects were seen. At the end of the first month, survived animals were sacrificed after anesthesia with intravenous pentobarbital sodium. Excised liver specimens were fixed in 10% buffered formalin for at least 2 days and PAS stating was performed after thin sectioning. PAS stained sections were studied with light microscopic scanning. All animal experiments were approved by the local animal ethics committee of Tabriz University of Medical Sciences, Tabriz, Iran.
Image analysis
The area measurements of hepatocytes glycogen store were made using light microscope in liver lobules of control group and rabbits treated with C.C pulp. Whereas, for diabetic rabbits treated with C.C seed measurements were performed near the portal area (periportal) and in the centrilobular regions of the hepatic lobules. Quantitative image analysis was performed using image analysis software (Image J).
Statistical analysis
All data were processed using either Microsoft Excel or Prism GraphPad software packages for statistical evaluation. Results were expressed as mean ± SD. Results were analyzed for statistical significance by one way ANOVA followed by Tukey's multiple comparisons test. All tests were done at the 5% level of significance and statistical significance was defined as P < 0.05.
RESULTS
In our experiment, after one day all animals that received 200 mg/kg/day of C.C pulp died. Among animals that received 100 mg/Kg/day of C.C pulp, only 50% (three of six rabbits) survived. There was no mortality among animals treated with 100 mg/kg/day of pulp and 100 or 200 mg/kg/day of C.C seed. Optical density of hepatocytes glycogen stores in periportal and centrilobular regions in normal [Figures 1 and 2] and diabetic rabbits are given in [Figures 3 and 4]. Hepatocytes glycogen stores of alloxanized rabbits that received 100 mg/kg of pulp of C.C showed a significant increase in comparison to diabetic control (99.87 ± 2.65 vs. 120.89 ± 3.15, n = 25, P < 0.0001) [Figures 5 and 6]. Application of pulp and seed extract of C.C in alloxanized rabbits was associated with re-appearance of glycogen in hepatocytes. Deposited glycogen in seed-treated diabetic animals was significantly more in centrilobular rather than periportal hepatocytes (89.19 ± 5.16 vs. 128.95 ± 1.33, n = 25, P < 0.0001) [Figures 7 and 8]. Congestion was observed in sinusoid and central veins of pulp treated animals [Figure 6]. Dose escalation of seed has no effect on the distribution pattern of accumulated glycogen
Figure 1.

Photomicrograph of the liver of control rabbits. Hepatocytes were full of glycogen. CV; central vein, PS; Portal space. PAS staining. ×330
Figure 2.

Photomicrograph of liver section of control rabbit. Glycogen granules (arrow) in hepatocytes (H) stained with PAS. ×1650
Figure 3.

Photomicrograph of the liver of diabetic control rabbit. There were on glycogen in some of hepatocytes (arrow). CV; central vein, PS; Portal space. PAS staining. ×330
Figure 4.
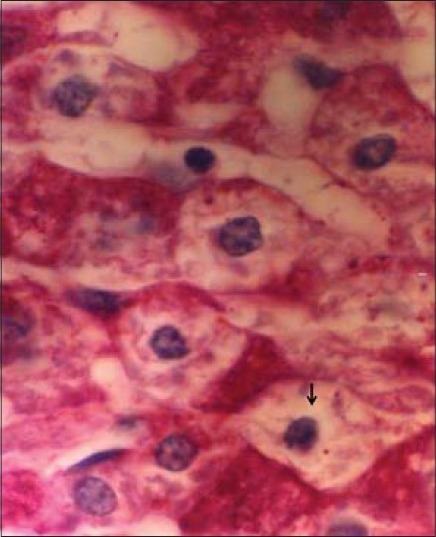
Photomicrograph of liver section of diabetic rabbit. There was no glycogen in hepatocyte (arrow). PAS staining. ×1650
Figure 5.

Photomicrograph of liver section of diabetic rabbit after treatment with pulp extract of citrullus colocynthis (100 mg/kg/day). Glycogen deposited in all hepatocytes again. Congestion in central vein (arrow). CV; central vein, PS; Portal space. PAS staining. ×330
Figure 6.

Photomicrograph of liver section of diabetic rabbit after treatment with pulp extract of citrullus colocynthis (100 mg/kg/day). Glycogen filled hepatocytes. S; sinusoid PAS staining. ×165
Figure 7.

Photomicrograph of liver section of diabetic rabbit after treatment with seed extract of Citrullus Colocynthis (100 mg/kg/day). Peripheral hepatocytes of hepatic lobule lost glycogen more than central hepatocytes around central vein (CV). PS; Portal space. PAS staining. ×330
Figure 8.

Photomicrograph of liver section of diabetic rabbit after treatment with seed extract of Citrullus Colocynthis (100 mg/kg/day). Histologic structure is the same as control. CV; central vein, S; sinusoid. PAS staining. ×1650
DISCUSSION
Our data shows a significant reduction in the amount of hepatocytes glycogen stores in C.C treated animals. No side effect has been seen. Dose escalation showed no significant benefit. Interestingly, the pattern of glycogen deposition in classic lobules of liver in seed-treated group was different from pulp-treated group [Figures 3 and 4]. Distribution pattern of PAS positive granules in classic lobules of liver was partially uniform in pulp-treated diabetic animals; similar to healthy control animals.
Cucurbitaceae seeds are traditionally used as antidaibetic agents in Mediterranean countries.[17] The aqueous extract of C.C, administrated via oral rout was associated with partly amelioration of some of the toxic effects of streptozotocin-induced diabetes in animals.[6] It was also able to reduce plasma glucose levels in normal rabbits after 1 h, and significantly after 2, 3, and 6 hrs.[7] The rind of C.C and its aqueous extract contains phenolics, Flavonoids, tertiary and quaternary alkaloids, glycosides and saponin compounds, as revealed by photochemical screening.[7,18] The hypoglycemic effect of the aqueous extract of C.C is mainly attributed to its saponin fraction.[7] Rather than its effects on the plasma glucose, orally administrated extract of C.C has been shown to affect some other parameters of DM, as pronounced reduction of HbA1c in type II diabetic patients.[17] In these cases improvement in glycemic profile was seen without any notable adverse effect.[17]
Despite of the above mentioned antidaibetic actions of C.C, the underlying mechanisms of action are not clearly defined. In our experiment, depleted glycogen stores of diabetic rabbits are an important finding which may be attributable to the activation of glucokinase enzyme.[19] This Insulin-dependent enzyme activation may explain the hypoglycemia seen by the medicinal herbs in indicative diabetic cases.[19] The seed extract of C.C has been demonstrated to have insulinotropic responses.[15] Such an effect may be possible in the case of pulp extract of C.C, but other mechanisms of action have been suggested for the hypoglycemic effects of Cucurbitaceae family. Decreased absorption of glucose via intestinal mucosa, decreased re-absorption of glucose from proximal renal tubule, antiglucagonic effect, altered metabolism of glucose by modification of the involved enzymes and an agonist of insulin secretion have been proposed for these effects.[20,21]
The increased glycogen deposition seen in the rabbits treated with pulp and seed extracts of C.C is an indirect measure of the increased glycogenesis and decreased glycogenolysis and gluconeogensis. Some part of the antidaibetic action of C.C may be explainable by the altered glycogen metabolism. This altered glycogen metabolism may also be seen with other medicinal herbs applied in the treatment of diabetes, but need to be evaluated by further studies. The different distribution pattern of glycogen accumulation in diabetic rabbit treated with pulp and seed extract of C.C seems interesting, but its importance needs to be determined. Despite of the clear role of the C.C in the treatment of diabetes, clarification of the underlying mechanisms may be helpful for the development of new therapies for DM, a disease with high morbidity.
In conclusion the anti-diabetic effects of C.C can be explained by its effects on accumulation of glycogen stores in hepatocytes. The importance of varied sites of glycogen deposition by the application of C.C needs to be determined.
ACKNOWLEDGMENT
Authors acknowledge the research vice chancellor of Tabriz University of Medical Science, Pharmacognesia Dept. of Pharmacy faculty and histology Dept. of Medical faculty.
Limitations of the study
Our data demonstrates the mechanism of action of C.C by its effects on reduction of glycogen storage. The principal part of the C.C for this action is its pulp. The limitation of our study is lack of human investigation to show the benefits of C.C on human glycogen storage. If C.C shows relevant effects on glycogen storage, we suggest a clinical trial on human population or further study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: Blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 2.Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13:1203–18. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- 3.Feng CG, Zhang LX, Liu X. Progress in research of aldose reductase inhibitors in traditional medicinal herbs. Zhongguo Zhong Yao Za Zhi. 2005;30:1496–500. [PubMed] [Google Scholar]
- 4.Vuksan V, Sievenpiper JL. Sievenpiper, Herbal remedies in the management of diabetes: Lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dis. 2005;15:149–60. doi: 10.1016/j.numecd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Huseini HF, Darvishzadeh F, Heshmat R, Jafariazar Z, Raza M, Larijani B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of type II diabetic patients: A randomized, double blind, placebo-controlled clinical trial. Phytother Res. 2009;23:1186–9. doi: 10.1002/ptr.2754. [DOI] [PubMed] [Google Scholar]
- 6.Al-Ghaithi F, El-Ridi MR, Adeghate E, Amiri MH. Biochemical effects of Citrullus colocynthis in normal and diabetic rats. Mol Cell Biochem. 2004;261:143–9. doi: 10.1023/b:mcbi.0000028749.63101.cc. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Hassan IA, Abdel-Barry JA, Tariq Mohammeda S. The hypoglycaemic and antihyperglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J Ethnopharmacol. 2000;71:325–30. doi: 10.1016/s0378-8741(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 8.Delazar A, Gibbons S, Kosari AR, Nazemiyeh H, Modarresi M, Nahar L. Flavone c-glycosides and cucurbitacin glycosides from citrullus colocynthis. DARU. 2006;14:109–14. [Google Scholar]
- 9.Adam SE, Al-Yahya MA, Al-Farhan AH. Response of Najdi sheep to oral administration of Citrullus colocynthis fruits, Nerium oleander leaves or their mixture. Small Rumin Res. 2001;40:239–44. doi: 10.1016/s0921-4488(01)00184-5. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa M, Morikawa T, Kobayashi H, Nakamura A, Matsuhira K, Nakamura S, et al. Bioactive saponins and glycosides. XXVII. Structures of new cucurbitane-type triterpene glycosides and antiallergic constituents from Citrullus colocynthis. Chem Pharm Bull (Tokyo) 2007;55:428–34. doi: 10.1248/cpb.55.428. [DOI] [PubMed] [Google Scholar]
- 11.Tannin-Spitz T, Bergman M, Grossman S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem Biophys Res Commun. 2007;364:181–6. doi: 10.1016/j.bbrc.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 12.Jayaprakasam B, Seeram NP, Nair MG. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett. 2003;189:11–6. doi: 10.1016/s0304-3835(02)00497-4. [DOI] [PubMed] [Google Scholar]
- 13.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 14.Arena JM, Drew RH. 5th ed. Illinois: Springfield, Thomas; 1980. Poisoning: Toxicology, symptoms, treatments. [Google Scholar]
- 15.Nmila R, Gross R, Rchid H, Roye M, Manteghetti M, Petit P, et al. Insulinotropic effect of Citrullus colocynthis fruit extracts. Planta Med. 2000;66:418–23. doi: 10.1055/s-2000-8586. [DOI] [PubMed] [Google Scholar]
- 16.Platel K, Srinivasan K. Plant foods in the management of diabetes mellitus: Vegetables as potential hypoglycaemic agents. Nahrung. 1997;41:68–74. doi: 10.1002/food.19970410203. [DOI] [PubMed] [Google Scholar]
- 17.Huseini HF, Darvishzadeh F, Heshmat R, Jafariazar Z, Raza M, Larijani B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of type II diabetic patients: A randomized, double blind, placebo-controlled clinical trial. Phytother Res. 2009;23:1186–9. doi: 10.1002/ptr.2754. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Kumar D, Manjusha, Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008;58:215–21. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- 19.Baltrusch S, Langer S, Massa L, Tiedge M, Lenzen S. Improved metabolic stimulus for glucose-induced insulin secretion through GK and PFK-2/FBPase-2 coexpression in insulin-producing RINm5F cells. Endocrinology. 2006;147:5768–76. doi: 10.1210/en.2006-0694. [DOI] [PubMed] [Google Scholar]
- 20.Khan BA, Abraham A, Leelamma S. Hypoglycemic action of Murraya-Koenigii (Curry Leaf) and Brassica-Juncea (Mustard)-mechanism of action. Indian J Biochem Biophys. 1995;32:106–8. [PubMed] [Google Scholar]
- 21.Chandrasekar B, Bajpai MB, Mukherjee SK. Hypoglycemic activity of Swertia chirayita (Roxb ex Flem) Karst. Indian J Exp Biol. 1990;28:616–8. [PubMed] [Google Scholar]


