Figure 3.
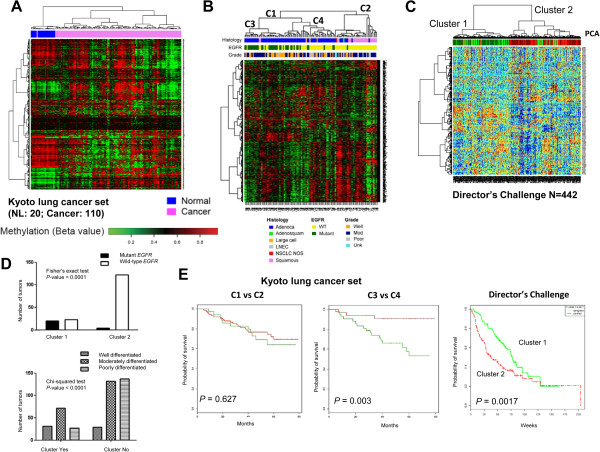
NSCLC SRAMs are differentially methylated between normal and tumor tissues segregate into molecular pathologic subtypes. A) Heatmap of the hierarchical clustering of 637 matched probes for SRAMs in the Kyoto set of 110 tumors and compared to 20 unmatched normal lung tissue samples. B) Using matched 637 probes from the HumanMethylation450 to the HumanMethylation27 platforms, unsupervised clustering of 110 tumors from the Kyoto tumor set was conducted, with tumor clustering along lines of histology (adenocarcinoma vs. squamous cell carcinoma), EGFR mutation status, and tumor grades. C-D) Unsupervised clustering based on gene expression of the matched gene regulatory module (GRM) genes on the Director’s Challenge dataset of 442 lung adenocarcinomas. The GRMs were able to segregate the tumors in the Director’s Challenge into two main groups, with EGFR mutations and well-to-moderately differentiated tumors in group 1, and wild-type EGFR and moderately-to-poorly differentiated tumors in group 2. E) SRAM segregates NSCLC tumors into prognostic groups in the Kyoto and Director’s Challenge tumor sets. Branch point C1 vs C2 mostly segregates squamous from adenocarcinoma, without a difference in prognosis; whereas branch point C3 vs. C4 is highly prognostic because of the separation of EGFR mutant from wild-type tumors. For the Director’s Challenge tumor set, the two clusters that preferentially separate EGFR mutant and wild-type tumors are also prognostic for survival.
