Figure 3.
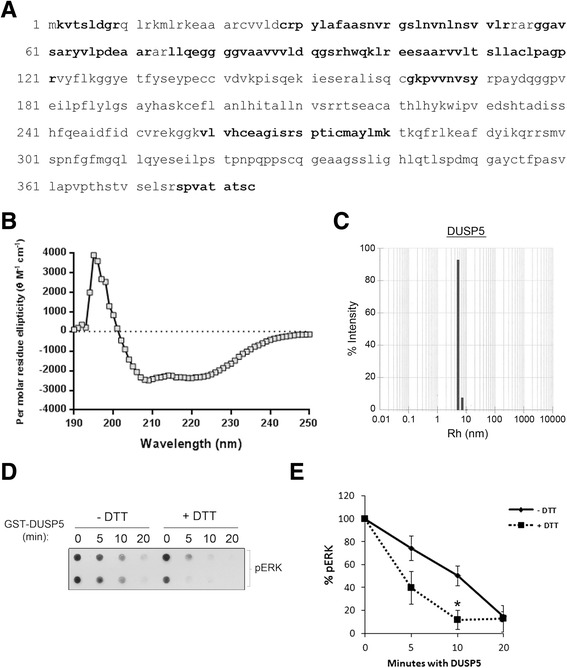
Protein characterization. (A) Mass spectrometric analysis was performed by Bioproximity, LLC on coomassie identified bands of the appropriate size separated by SDS-PAGE. Samples were subjected to in-gel trypsin digestion and the peptides recovered for mass spectrometric analysis. (B) Circular dichroism was performed to determine folding and secondary structure of DUSP5, which was found to be 43% alpha helical and 20% beta-sheet. (C) Dynamic Light Scattering predicts oligomerization of both DUSP5 and S147P. (D & E) GST-DUSP5 is active and sensitive to the addition of DTT. Specifically, 1 mM DTT increased the activity of GST-DUSP5 against pERK2 in vitro.
