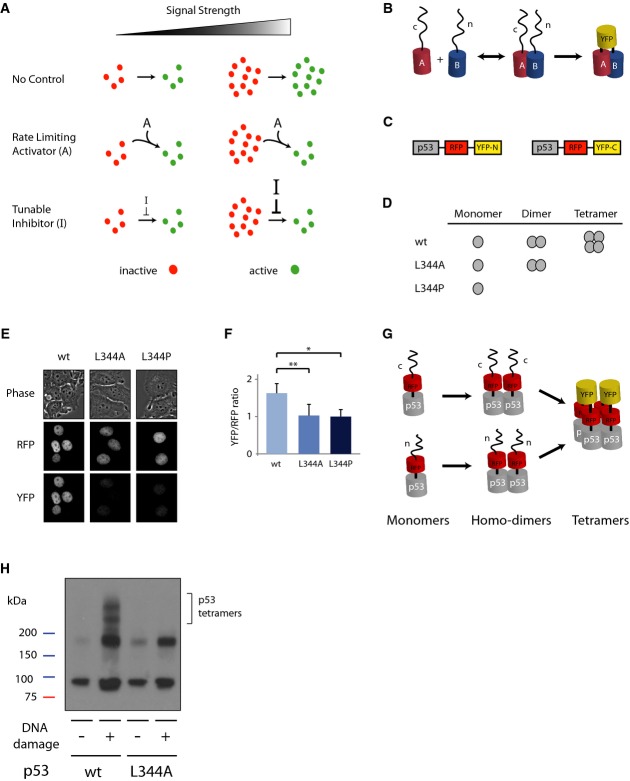
Schematic drawing of potential mechanisms constraining the levels of an active molecule. In all
cases, the levels of an inactive molecule (red) are proportional to the signal strength. In the
absence of a control system (top), the levels of the active form (green) are also proportional to
the signal strength. The linear relationship between signal strength and molecule activation can be
broken by the presence of a constant activator “A”, which limits the rate of
activation (middle), or by the presence of a tunable inhibitor “I”, the strength of
which depends on the strength of the signal (bottom).
PCA is based on tagging putative interactive proteins (“A” and “B”)
with two different fragments of a fluorescence protein (“n” and “c”).
The interaction between A and B brings the unfolded non-fluorescent fragments in close proximity and
they form a full fluorescent protein.
Schematic drawing of the p53 reporters.
p53 species; p53 L344A forms dimers but not tetramers, while p53 L344P is only monomeric.
Images of cells expressing the constructs in (C) with mutant or wild-type p53s in bright-field
illumination, RFP and YFP.
Ratio of YFP to RFP fluorescence level in cells. Median and standard deviation are reported, and
values are normalized to p53 L344P (n ≥ 50,
*P = 10−17,
**P = 10−14, p53 L344A and p53
L344P are not statistically significantly different P = 0.22;
P-values obtained by Mann–Whitney U-test).
p53 forms homo-dimers, in which both monomers are tagged with the same fragment of YFP leading to
no YFP signal. When dimers form tetramers the split YFP protein is formed and becomes
fluorescent.
Lysate crosslinking with 0.025% glutaraldehyde with or without DNA damage induction by NCS
(400 ng/mL) on p53 wild-type and p53 L344A dimeric mutant tagged with YFP PCA reporter system
(C).