Abstract
Background
It has been suggested that adverse postoperative outcomes may have a negative impact on longterm survival in patients with colorectal liver metastases.
Objectives
This study was conducted to evaluate the prognostic impact of postoperative complications in patients submitted to a potentially curative resection of colorectal liver metastases.
Methods
A retrospective analysis of outcomes in 199 patients submitted to hepatic resection with curative intent for metastatic colorectal cancer during 1999–2008 was conducted.
Results
The overall complication rate was 38% (n = 75). Of all complications, 79% were minor (Grades I or II). There were five deaths (3%). The median length of follow-up was 39 months. Rates of 5-year overall and disease-free survival were 44% and 27%, respectively. Univariate analysis demonstrated that an elevated preoperative level of carcinoembryonic antigen (CEA), intraoperative blood loss of >300 ml, multiple metastases, large (≥35 mm) metastases and resection margins of <1 mm were associated with poor overall and disease-free survival. In addition, male sex and synchronous metastases were associated with poor disease-free survival. Postoperative complications did not have an impact on either survival measure. The multivariate model did not include complications as a predictive factor.
Conclusions
Postoperative complications were not found to influence overall or disease-free survival in the present series. The number and size of liver metastases were confirmed as significant prognostic factors.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in Australia, where approximately 4000 patients die from this disease each year (age-standardized mortality is approximately 18%).1 The liver is the most common site for metastatic disease, and resection of isolated metastases prolongs survival and achieves cure in selected patients.2–4 Operative mortality following liver resection is <5% in high-volume centres,5–8 but postoperative morbidity rates remain as high as 56%.9–11
Numerous preoperative factors have been shown to predict poor longterm outcomes following the resection of colorectal liver metastases. These include an elevated serum carcinoembryonic antigen (CEA) level, poor primary tumour differentiation and primary tumour lymph node involvement.9 It has also been suggested that postoperative complications following liver resection may have a negative impact on longterm survival.11 However, the mechanisms of this putative relationship are unclear.
The purpose of this study was to evaluate the prognostic impact of postoperative complications, as defined by the Clavien–Dindo system for grading complications,12 in patients submitted to resection for colorectal liver metastases. A secondary aim was to determine additional factors that might predict poor longterm survival in these patients.
Materials and methods
A retrospective analysis of prospectively collected data was performed. The study sample consisted of consecutive patients submitted to first-time liver resection with curative intent for colorectal liver metastases at Royal North Shore Hospital and associated campuses during the study period of 1999–2008. Ethics approval for the study was provided by the Human Research Ethics Committee of the Northern Sydney Area Health Service.
Data collection and definitions
All data were collected prospectively and extracted from the departmental liver database for retrospective analysis. Additional information was obtained from the hospital or the consulting surgeon's records. A range of demographic, clinical, radiological, pathological and follow-up variables were assessed.
Synchronous liver metastases were defined as those presenting within 4 months of the primary CRC diagnosis; metachronous liver metastases were defined as those identified at >4 months after the primary CRC diagnosis.
Intraoperative data collected included the total duration of inflow occlusion (Pringle manoeuvre), the estimated volume of blood loss, and the transfusion of blood products. Liver resection nomenclature was documented as per the Brisbane terminology.13 Minor liver resections were defined as those in which up to two Couinaud liver segments were removed and major liver resections were defined as those in which three or more Couinaud liver segments were removed.
All perioperative morbidity was recorded in a prospective manner. All complications recorded were reviewed weekly at the unit meeting. These complications were categorized using the Clavien–Dindo system of classification.12 The highest grade complication was recorded for each patient. Postoperative liver failure, postoperative bleeding and postoperative bile leakage were documented using standard definitions as per the International Study Group of Liver Surgery.14–16
Perioperative mortality referred to death during the same admission (in-hospital) or within 90 days of surgery. For survival analyses, patients were divided into two groups consisting of: (i) those patients requiring intervention (complications of Clavien–Dindo Grade II or higher), and (ii) those patients who either had no postoperative complications or had complications that did not require intervention (Clavien–Dindo Grade I). Specifically, patients who died in the perioperative period (Clavien–Dindo Grade V complication) were excluded from the survival analyses because otherwise death would represent both a predictor and an outcome measure in this group. Overall survival (OS) was defined as the time from hepatic surgery to the date of death (all-cause mortality). Disease-free survival (DFS) was defined as the time from hepatic surgery to the date of either death or the first evidence of recurrence (local, regional or metastatic). Follow-up time was taken as the time from hepatic surgery to the date of last follow-up. Follow-up and survival times were recorded in months.
Histopathological findings in the primary CRC and the liver metastases were obtained from hospital pathology reports. For the primary cancer, these data included the location and differentiation of the tumour, and lymph node status. For liver metastases, these data included the size of the largest tumour, the number of metastases and the tumour differentiation. Patients were stratified according to liver resection margins for the purpose of analysis: R2 represented a margin with macroscopic involvement; R1 represented a margin with microscopic involvement, and R0 represented a negative margin. Margins were measured as <1 mm, 1–10 mm and >10 mm.
Preoperative workup and operative technique
All patients underwent a baseline preoperative assessment that included liver function tests, coagulation studies, serum CEA levels and a fine-cut, multi-phase computed tomography (CT) scan of the abdomen and thorax. From January 2004, a positron emission tomography (PET) scan was also performed to exclude extrahepatic disease. All patients were discussed at a multidisciplinary group meeting prior to liver resection. Operative criteria included the likelihood of achieving an R0 resection (microscopically clear margin) along with the preservation of vascular inflow and outflow and an adequate post-resection liver remnant volume. Patients with limited extrahepatic intra-abdominal disease (e.g. portahepatis lymph node involvement or isolated upper quadrant peritoneal disease) were not excluded from resection. Liver transection was performed using the Cavitron Ultrasonic Surgical Aspirator (CUSA) dissection device (Integra LifeSciences Corp., Plainsboro, NJ, USA) under low central venous pressure conditions with intermittent inflow occlusion.
The follow-up regime included 6-monthly clinical evaluations, assessment of serum tumour markers and annual CT scans of the thorax and abdomen. Triple-phase contrast-enhanced magnetic resonance imaging was performed if clinically indicated. Patients were followed up annually indefinitely after the initial 5-year follow-up.
Statistical analysis
Demographic descriptive statistics were reported using the mean ± standard deviation (SD) and median [interquartile range (IQR)] depending on the distribution. Kaplan–Meier curves with 95% Greenwood bands were constructed for overall and disease-free survival. Patients with Grade V complications (perioperative patient death) were excluded from survival analyses.
Inferential univariate survival analysis was performed using the log-rank test after the conversion of variables into categorical variables. The cut-off value selected for these categorical variables either represented a clinically relevant quantity or was used to divide the groups into equal binary groups. Multivariate analysis was performed by constructing Cox proportional hazards models from potentially significant covariates identified in the univariate analysis (P <0.2). The purposeful selection of covariates method was used to select variables for the final model (i.e. stepwise removal whereby covariates thought to be clinically significant were retained if necessary). The final model was then assessed for the validity of the proportional hazards assumption using Shoenfeld residuals and goodness-of-fit using Cox–Snell residuals. Data management and statistical analyses were performed using stata SE for Windows Version 11.2 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
During the study period of 1999–2008, 224 patients underwent resection for colorectal liver metastases. Ten of these patients (4%) underwent laparoscopy or laparotomy only because of unsuspected intra-or extrahepatic disease. Fifteen patients underwent a repeat liver resection during the study period (14 had one repeat resection and one had two repeat resections). The remaining 199 patients underwent a first-time liver resection with curative intent. These patients are the subject of this study.
The clinicopathological characteristics of the study group are outlined in Table 1.
Table 1.
Characteristics of 199 patientsa submitted to hepatic resection with curative intent for metastatic colorectal cancer
| Variable | ||
|---|---|---|
| Demographic characteristics | ||
| Gender, male, n (%) | 132 (66%) | |
| Age, years, mean ± SD | 63 ± 11 | |
| Temporal relationship, metachronous, n (%) | 107 (54%) | |
| Colon to hepatic surgery, months, median (IQR) (n = 192) | 12 (5–23) | |
| CEA, μg/l, median (IQR) (n = 159) | 8 (4–27) | |
| Elevated CEA, n (%) | 124 (78%) | |
| Perioperative characteristics | ||
| Resection, major, n (%) | 120 (60%) | |
| Blood loss of >300 ml, n (%) (n = 195) | 68 (35%) | |
| Transfusion, n (%) (n = 189) | 29 (15%) | |
| Pringle duration >15 min, n (%) (n = 145) | 112 (77%) | |
| Operating time, min, median (IQR) (n = 190) | 198 (150–266) | |
| No complications, n (%) | 123 (62%) | |
| Clavien–Dindo grade, n (%) (n = 198) | ||
| Grade I | 17 (9%) | |
| Grade II | 42 (21%) | |
| Grade IIIa | 5 (3%) | |
| Grade IIIb | 3 (2%) | |
| Grade IVa | 2 (1%) | |
| Grade IVb | 1 (1%) | |
| Grade V | 5 (3%) | |
| Primary tumour characteristics | ||
| Location: colon, n (%) | 141 (71%) | |
| Location: rectum, n (%) | 58 (29%) | |
| Primary differentiation, poor, n (%) (n = 181) | 27 (15%) | |
| Lymph node status positive, n (%) (n = 192) | 121 (63%) | |
| Liver metastases characteristics | ||
| Margin, mm, median (IQR) (n = 197) | 3 (1–10) | |
| Margin R1 (0 mm), n (%) | 38 (19%) | |
| Margin <1 mm, n (%) | 22 (11%) | |
| Margin 1–10 mm, n (%) | 94 (48%) | |
| Margin >10 mm, n (%) | 43 (22%) | |
| Number of metastases, median (IQR) (n = 196) | 2 (1–2) | |
| Size of metastases, mm, median (IQR) (n = 195) | 35 (24–57) | |
Numbers available for analysis vary as shown.
SD, standard deviation; IQR, interquartile range; CEA, carcinoembryonic antigen.
The majority of patients (71%) had a primary colon cancer and 63% (121/192) of the whole cohort had node-positive primary tumours. Overall, 54% of patients had metachronous liver metastases and half of these underwent liver surgery within 1 year of the primary cancer resection. Nine patients (5%) submitted to a simultaneous hepatic and colorectal resection and four patients (2%) underwent a liver resection before bowel surgery.
The overall complication rate was 38%. The majority of complications were minor (Grades I and II). Major complications (Grades III and IV) occurred in 11 patients (6%). Five patients died during the postoperative period, giving an overall operative mortality of 3%.
The median resection margin in all patients was 3 mm, which reflects a parenchyma-sparing operative approach. An R0 resection was achieved in 159 (81%) patients and an R1 resection occurred in the remaining patients (n = 38, 19%). There were no R2 resections in this series.
Overall and disease-free survival
Median follow-up in these patients was 39 months (IQR: 19–64 months). Overall survival outcomes are documented in Fig. 1. During the study period, the total number of deaths in the cohort was 107 (54%) and the total time at risk amounted to 8827 months. Median survival was 48 months [95% confidence interval (CI) 42–65] and 5-year OS was 44% (95% CI 37–52%).
Figure 1.
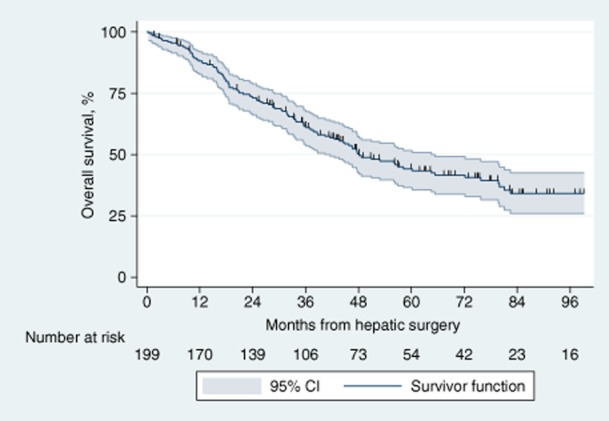
Kaplan–Meier curve for overall survival in 199 patients submitted to hepatic resection with curative intent for metastatic colorectal cancer. 95% CI, 95% confidence interval
Disease-free survival is shown in Fig. 2. The total number of deaths or recurrences in the cohort was 143 (72%) and the total time at risk amounted to 5707 months. Median DFS was 17 months (95% CI 12–23). Five-year DFS was 27% (95% CI 21–34%).
Figure 2.
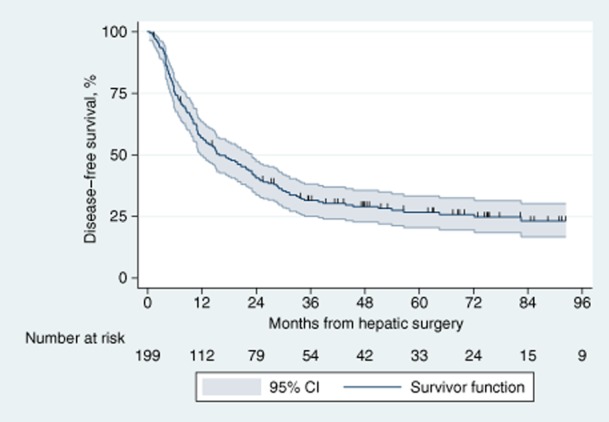
Kaplan–Meier curve for disease-free survival in 199 patients submitted to hepatic resection with curative intent for metastatic colorectal cancer. 95% CI, 95% confidence interval
Univariate analysis
The univariate analysis for overall and disease-free survival is presented in Table 2. Patients with a resection margin of <1 mm had a worse outcome compared with those with a larger margin (≥1 mm), with median survival of 39 months and 57 months, respectively (P = 0.025). However, there was no statistically significant difference between the R0 (<1 mm) and R1 groups (median survival of 39 months in both groups; P = 0.679), which may explain the lack of any statistically significant difference when R1 patients were compared with R0 (any margin) patients (median survival: 39 months and 56 months, respectively; P = 0.133).
Table 2.
Summary of univariate analyses of overall survival (OS) and disease-free survival (DFS) performed by log-rank test in 199 patients submitted to hepatic resection with curative intent for metastatic colorectal cancer
| Variable | n | OS, months, median | P-value | DFS, months, median | P-value |
|---|---|---|---|---|---|
| Sex | 0.066 | 0.010 | |||
| Female | 67 | 83 | 25 | ||
| Male | 132 | 46 | 15 | ||
| Age | 0.089 | 0.188 | |||
| <64 years | 99 | 65 | 22 | ||
| ≥64 years | 100 | 43 | 12 | ||
| Temporal relationship | 0.162 | 0.024 | |||
| Synchronous | 92 | 44 | 11 | ||
| Metachronous | 106 | 57 | 23 | ||
| Colon to hepatic surgery | 0.975 | 0.345 | |||
| ≤ 12 months | 98 | 49 | 15 | ||
| >12 months | 93 | 48 | 21 | ||
| Carcinoembryonic antigen | 0.023 | 0.067 | |||
| Normal | 35 | Not reached | 29 | ||
| Elevated | 124 | 45 | 14 | ||
| Resection | 0.390 | 0.118 | |||
| Minor | 79 | 56 | 20 | ||
| Major | 119 | 48 | 16 | ||
| Blood loss | 0.026 | 0.012 | |||
| ≤ 300 ml | 127 | 58 | 20 | ||
| >300 ml | 68 | 39 | 15 | ||
| Transfusion | 0.178 | 0.206 | |||
| No | 159 | 51 | 18 | ||
| Yes | 29 | 36 | 13 | ||
| Pringle duration | 0.303 | 0.161 | |||
| ≤ 15 min | 33 | 60 | 22 | ||
| >15 min | 111 | 47 | 12 | ||
| Operating time | 0.647 | 0.238 | |||
| <180 min | 63 | 48 | 15 | ||
| 180–239 min | 59 | 53 | 15 | ||
| ≥240 min | 67 | 48 | 17 | ||
| Complications, Clavien–Dindo | 0.877 | 0.658 | |||
| None or Grade I | 140 | 51 | 17 | ||
| Grades II–IV | 53 | 49 | 18 | ||
| Grade V, death | 5 | Excluded | |||
| Any complication (death excluded) | 0.552 | 0.310 | |||
| None | 123 | 56 | 17 | ||
| Any | 70 | 45 | 18 | ||
| Primary cancer | 0.053 | 0.450 | |||
| Colon | 141 | 60 | 18 | ||
| Rectum | 57 | 44 | 15 | ||
| Primary differentiation | 0.503 | 0.645 | |||
| Good/moderate | 153 | 48 | 17 | ||
| Poor | 27 | 76 | 10 | ||
| Lymph node status | 0.105 | 0.172 | |||
| Negative | 71 | 56 | 23 | ||
| Positive | 120 | 45 | 15 | ||
| Margin | |||||
| R1 (0 mm) | 38 | 39 | 11 | ||
| R0 (<1 mm) | 21 | 39 | 9 | ||
| 1–10 mm | 94 | 56 | 17 | ||
| >10 mm | 43 | 60 | 45 | ||
| <1 versus ≥1 | 0.025 | <1 versus ≥1 | 0.001 | ||
| R1 versus any R0 | 0.133 | R1 versus any R0 | 0.004 | ||
| Number of metastases | 0.028 | <0.001 | |||
| Solitary | 91 | 58 | 28 | ||
| Multiple | 104 | 39 | 11 | ||
| Size of metastases | 0.003 | 0.005 | |||
| <35 mm | 91 | 60 | 25 | ||
| ≥35 mm | 103 | 36 | 12 |
Patients with postoperative complications requiring intervention (Clavien–Dindo Grade II or higher) were compared with those with Grade I or no complications in terms of overall and disease-free survival. There were no statistically significant differences for either outcome measure. This is confirmed by the overlapping Kaplan–Meier curves demonstrated in Figs 3 and 4.
Figure 3.
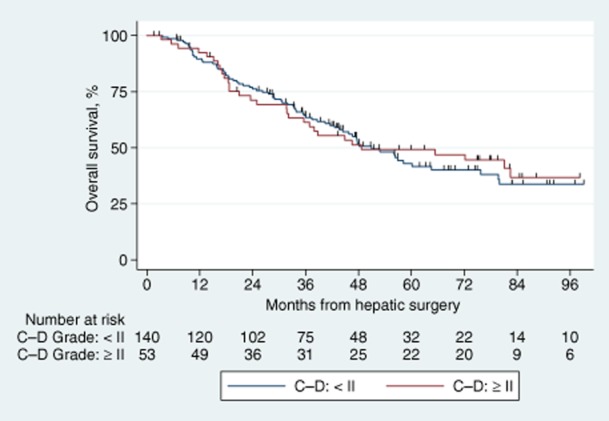
Kaplan–Meier survival curves comparing overall survival in patients with and without complications. C–D, Clavien–Dindo
Figure 4.
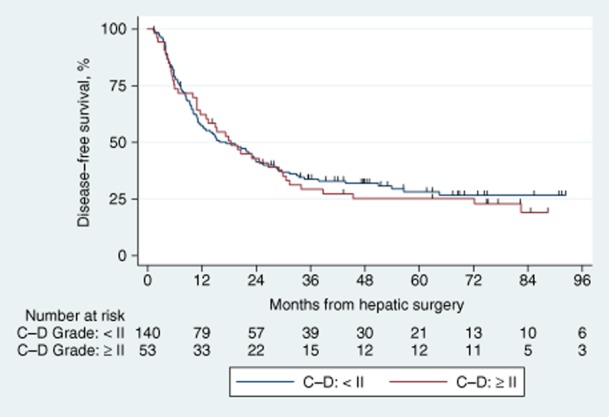
Kaplan–Meier survival curves comparing disease-free survival in patients with and without complications. C–D, Clavien–Dindo
Multivariate analysis
The final modes for OS and DFS are shown in Table 3. Large and multiple metastases are important factors associated with poorer OS and DFS.
Table 3.
Final multivariate model for overall and disease-free survival in 199 patients submitted to hepatic resection with curative intent for metastatic colorectal cancer
| Variable | HR | 95% CI | SE | Z-value | P-value | |
|---|---|---|---|---|---|---|
| Overall survival | ||||||
| Rectal versus colon primary | 1.42 | 0.94 | 2.14 | 0.30 | 1.68 | 0.093 |
| Size ≥35 mm | 2.04 | 1.36 | 3.05 | 0.42 | 3.45 | 0.001 |
| Multiple (versus solitary) | 1.82 | 1.22 | 2.71 | 0.37 | 2.94 | 0.003 |
| Disease-free survival | ||||||
| Sex | 1.60 | 1.10 | 2.31 | 0.30 | 2.49 | 0.013 |
| Size ≥35 mm | 1.97 | 1.40 | 2.79 | 0.35 | 3.84 | <0.001 |
| Multiple metastases (versus solitary) | 2.08 | 1.47 | 2.95 | 0.37 | 4.13 | <0.001 |
HR, hazard ratio; 95% CI, 95% confidence interval; SE, standard error.
Discussion
The development of a complication following major surgery may prolong a patient's hospital stay and is likely to increase overall hospital costs. Complications often result in an extended intensive care unit (ICU) admission, which has been shown to have a negative impact on longterm survival after both oncological and non-oncological surgery.17 In the present study, the relationship between postoperative morbidity and longterm outcome was examined in 199 patients who underwent resection of colorectal liver metastases. No relationship between the development of a postoperative complication and longterm survival was found.
Major morbidity (defined as a complication of Clavien–Dindo Grades III or IV) occurred in fewer than 6% of patients (n = 11/199). Five-year rates of OS and DFS in this series were 44% and 27%, respectively. These are similar to those in the published literature, although reported survival rates range widely from 9% to 63% for OS and from 4% to 47% for DFS.18
Postoperative morbidity has long been thought to be a prognostic factor in major surgery. In relation to hepatic resection for colorectal metastases, Laurent et al. published an early landmark study suggesting that postoperative complications adversely affected both disease-free and overall survival.11 However, this study was criticized for its failure to systematically define and classify postoperative complications. Several years later, Schiesser and colleagues used the Clavien–Dindo system12 to report morbidity and found a negative relationship.19 However, this analysis included postoperative deaths (Clavien–Dindo Grade V) in the complication group, which caused a bias towards rejection of the null hypothesis. In 2008, Vigano et al. reported a similar negative relationship between postoperative morbidity and survival in 125 patients submitted to liver surgery for colorectal metastases.20 Again, this finding was questioned because there was no formal definition or classification of complications and no reporting of the effect on recurrence or DFS.
In contrast with the findings cited above, two large studies failed to show an unequivocal relationship. Ito et al. found that the presence of postoperative complications was not an independent poor prognostic factor for either disease-specific or disease-free survival in 1067 patients.5 Only a subgroup analysis of patients with a low risk for recurrence (clinical risk scores of <3) demonstrated an adverse relationship for both measures of survival.9 Confusingly, Farid et al., in a cohort of 705 patients, found a slight survival disadvantage in terms of OS but not DFS. Interestingly, there was a more definitive association with worse survival when a secondary analysis of infective complications versus non-infective complications was undertaken.21 Unlike the study by Schiesser et al.,19 both of the last two studies excluded patients with Grade V complications (postoperative death).
More recently, in 2013 Mavros et al. examined the outcomes of 251 patients after liver resection and found a strong association between complications and both overall and progression-free survival.22 Although postoperative deaths were excluded from the analysis, the Kaplan–Meier chart suggested that at least two of 14 patients in the major complications group died within 30 days of surgery, which may well have influenced the conclusion of the study.
Evidence of an adverse effect of postoperative morbidity on longterm survival has been found in other tumour types including colonic, hepatocellular and oesophageal cancers.23–26 In an analysis of 105 951 patients from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, Khuri et al. showed that postoperative complications were associated with poor survival after eight different types of major surgery, including non-cancer operations.27 This suggests that the effect of postoperative morbidity on survival is likely to be multifactorial, rather than related only to altered longterm cancer behaviour. Other factors that might impact on medium-or longterm pathophysiological stress, such as infection, cardiorespiratory disease or even poor quality of life, may well be important. Khuri et al. identified two distinct survival curves in patients with and without postoperative complications with an inflection point occurring between 27–180 days postoperatively.27 This suggests that although the impact of complications seems to extend beyond the immediate postoperative period, the duration of this effect is, nonetheless, time-limited. This finding has implications for interpretation of the literature and may explain the variable results published to date. Specifically, the finding of an association between complications and survival depends on: (i) the definition of the postoperative mortality group and whether this group is excluded from analysis; (ii) the relative importance of the physiological insult compared with the cancer biology on survival, and (iii) the degree of the physiological insult of the complication (and therefore the timing of the inflection point). As a result, studies that include early postoperative deaths tend to show an association. Furthermore, the adverse effect of postoperative complications tends to be more prominent in subgroup analyses of patients at low risk for recurrence or in those with infective complications.5,21
Mechanistically, the effect of complications on survival may be related to the immunomodulatory effect of the associated systemic inflammatory response.27 Inflammation and inflammatory cytokine release [especially of tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and IL-1] also play important roles in carcinogenesis through their influence on tumour development and immune response.28 A second putative pathway to perioperative immunomodulation relates to perioperative blood transfusion.29–32 Interestingly, of the six studies investigating the effects on survival of postoperative complications, only that by Farid et al. found blood transfusion to be a significant factor associated with poor disease-free (but not overall) survival.5,11,19–22 Low rates of blood transfusion in modern case series (often of <20%) and different protocols for the transfusion of leuko-depleted products or γ-irradiated packed cells may be important confounding factors.
The present study also examined a range of other prognostic factors and found that large or multiple liver metastases were independent predictors of poor DFS and OS. Repeatedly, these factors have been shown to be associated with worse longterm survival and are often included in prognostic scoring systems.9,33–37
An important issue when analysing the impact of complications on longterm outcome is whether perioperative data were collected prospectively or retrospectively. Minor complications (Grades I or II) may be dismissed as part of the ‘normal’ postoperative course with retrospective analysis. A strength of the present study was the use of prospectively collected and classified complication data, which limits the chance of under-reporting. The morbidity and mortality rates after liver resection identified herein are comparable with those reported in the literature.5,11,19,21 One drawback of the present study is the fact that the event rate for complications was relatively small, and accordingly this analysis may be underpowered to detect small differences in outcome.
Conclusions
In summary, the current study does not support the theory that postoperative morbidity affects the longterm oncological outcome of patients undergoing liver resection for colorectal metastases. Unfortunately, evidence from the literature is inconsistent between different studies. In patients submitted to non-oncological major surgery, there is evidence that being critically ill postoperatively has a medium-to longterm impact on survival. There are also plausible underlying mechanisms to explain this effect. Although this relationship may also be true for patients undergoing resection of colorectal liver metastases, the magnitude of this effect may be limited.
Conflicts of interest
None declared.
References
- Australian Institute of Health and Welfare. ACIM (Australian Cancer Incidence and Mortality) Books. Canberra, ACT: AIHW; 2011. [Google Scholar]
- Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M. Analysis of prognostic factors influencing longterm survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- Bentrem DJ, Dematteo RP, Blumgart LH. Surgical therapy for metastatic disease to the liver. Annu Rev Med. 2005;56:139–156. doi: 10.1146/annurev.med.56.082103.104630. [DOI] [PubMed] [Google Scholar]
- Shah SA, Bromberg R, Coates A, Rempel E, Simunovic M, Gallinger S. Survival after liver resection for metastatic colorectal carcinoma in a large population. J Am Coll Surg. 2007;205:676–683. doi: 10.1016/j.jamcollsurg.2007.06.283. [DOI] [PubMed] [Google Scholar]
- Ito H, Are C, Gonen M, D'Angelica M, Dematteo RP, Kemeny NE. Effect of postoperative morbidity on longterm survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994–1002. doi: 10.1097/SLA.0b013e31816c405f. [DOI] [PubMed] [Google Scholar]
- Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- Blumgart LH, Fong Y. Surgical options in the treatment of hepatic metastasis from colorectal cancer. Curr Probl Surg. 1995;32:333–421. doi: 10.1016/s0011-3840(05)80012-7. [DOI] [PubMed] [Google Scholar]
- Abdalla EK, Vauthey JN. Improved survival after resection of colorectal liver metastases. J Clin Oncol. 2006;24:2679. doi: 10.1200/JCO.2005.05.2688. authors' reply 2680–2681. [DOI] [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani S, Michaelson JS, Hutter MM, Lancaster RT, Warshaw AL, Henderson WG. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1284–1292. doi: 10.1016/j.jamcollsurg.2007.02.067. [DOI] [PubMed] [Google Scholar]
- Laurent C, Sa Cunha A, Couderc P, Rullier E, Saric J. Influence of postoperative morbidity on longterm survival following liver resection for colorectal metastases. Br J Surg. 2003;90:1131–1136. doi: 10.1002/bjs.4202. [DOI] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R. Post-hepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB. 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers TK, Verhofstad MH, Moons KG, Leenen LP. Longterm survival after surgical intensive care unit admission: fifty percent die within 10 years. Ann Surg. 2011;253:151–157. doi: 10.1097/SLA.0b013e3181ff45df. [DOI] [PubMed] [Google Scholar]
- Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiesser M, Chen JW, Maddern GJ, Padbury RT. Perioperative morbidity affects longterm survival in patients following liver resection for colorectal metastases. J Gastrointest Surg. 2008;12:1054–1060. doi: 10.1007/s11605-007-0438-y. [DOI] [PubMed] [Google Scholar]
- Vigano L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Longterm survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP. Correlation between postoperative infective complications and longterm outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91–100. doi: 10.1097/SLA.0b013e3181bfda3c. [DOI] [PubMed] [Google Scholar]
- Mavros MN, de Jong M, Dogeas E, Hyder O, Pawlik TM. Impact of complications on longterm survival after resection of colorectal liver metastases. Br J Surg. 2013;100:711–718. doi: 10.1002/bjs.9060. [DOI] [PubMed] [Google Scholar]
- Chok KS, Ng KK, Poon RT, Lo CM, Fan ST. Impact of postoperative complications on longterm outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81–87. doi: 10.1002/bjs.6358. [DOI] [PubMed] [Google Scholar]
- McArdle CS, McMillan DC, Hole DJ. The impact of blood loss, obstruction and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg. 2006;93:483–488. doi: 10.1002/bjs.5269. [DOI] [PubMed] [Google Scholar]
- Rizk NP, Bach PB, Schrag D, Bains MS, Turnbull AD, Karpeh M. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198:42–50. doi: 10.1016/j.jamcollsurg.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on longterm outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559–2566. doi: 10.1245/s10434-007-9434-4. [DOI] [PubMed] [Google Scholar]
- Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of longterm survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. doi: 10.1097/01.sla.0000179621.33268.83. discussion 341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther. 2010;87:401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP. Influence of transfusions on perioperative and longterm outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. discussion 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson KR, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988;208:679–687. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes RN, Rogatko A, Brennan MF. The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg. 1991;214:107–113. doi: 10.1097/00000658-199108000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson B, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998;22:268–276. doi: 10.1007/s002689900381. discussion 276–277. [DOI] [PubMed] [Google Scholar]
- Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto T. A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11:79–83. doi: 10.1007/s00534-002-0778-7. [DOI] [PubMed] [Google Scholar]
- Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]


