FIGURE 1.
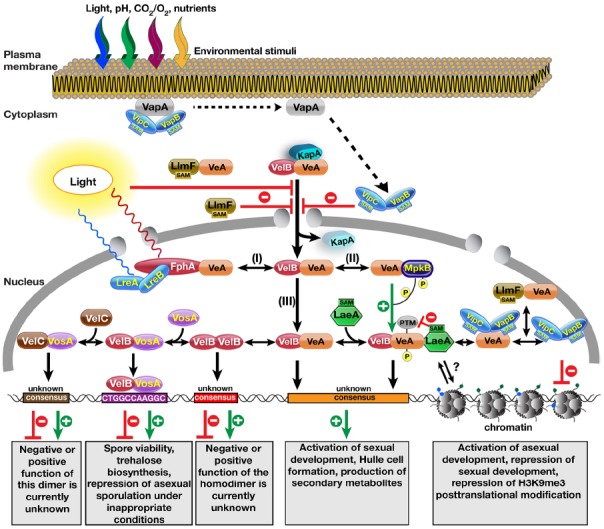
Molecular complexes formed by the velvet family proteins and the methyltransferases on the control of fungal development and secondary metabolite production. Nuclear entry of the VeA–VelB heterodimer is operated by the α-importin KapA protein. Light reduces nuclear entry of the heterodimer by an unknown mechanism. VapA tethers the two SAM-dependent methyltransferases to the plasma membrane. Reception of an unknown signal (e.g., light, pH, CO2, O2, starvation) triggers the release of VipC–VapB methyltransferase heterodimers, which are targeted to the nucleus. During their translocation to the nucleus, they inhibit the nuclear import of the VeA protein. Another LaeA-like SA\ependent methyltransferase LlmF also hinders the nuclear entry of the VeA protein and forms a complex with VeA. After entering the nucleus, the VelB–VeA dimer follows different options: (I) VeA interacts with the red light receptor protein phytochrome FphA, which together with blue light receptors LreA–LreB forms a tetrameric VeA–FphA–LreB–LreA complex. (II) VeA interacts with MAPK MpkB that phosphorylates VeA, which makes VeA more interactive for VelB. (III) VeA–VelB dimer is a part of more dynamic system where addition of methyltransferase LaeA results in the trimeric VelB–VeA–LaeA velvet complex. VelB–VeA or VelB–VeA–LaeA might bind to a consensus sequence to activate sexual developmental genes as well as SM gene clusters. Molecular function of LaeA between the chromatin and VelB–VeA heterodimer function is still unclear. VeA might recruit two methyltransferases VipC–VapB. Either VeA–VipC–VapB or VipC–VapB influence the histone posttranslational modifications (PTMs) and activates asexual genes. LlmF–VeA also forms a complex with VeA in the nucleus, whose function needs to be resolved. VelB component of VelB–VeA heterodimer dissociates from VeA by forming VelB–VelB homodimer. Free VelB also attracts the third velvet family protein VosA to form active transcription factor heterodimers that bind to target sequence of trehalose biosynthetic genes and asexual genes. Furthermore, VosA might recruit VelC, VosA–VelC heterodimer might activate the genes controlling sexual development and spore viability. Velvet family proteins might form more combinations of hetero and homodimer complexes as the Rel homology domain (RHD) proteins of NF-κB family in mammals.
