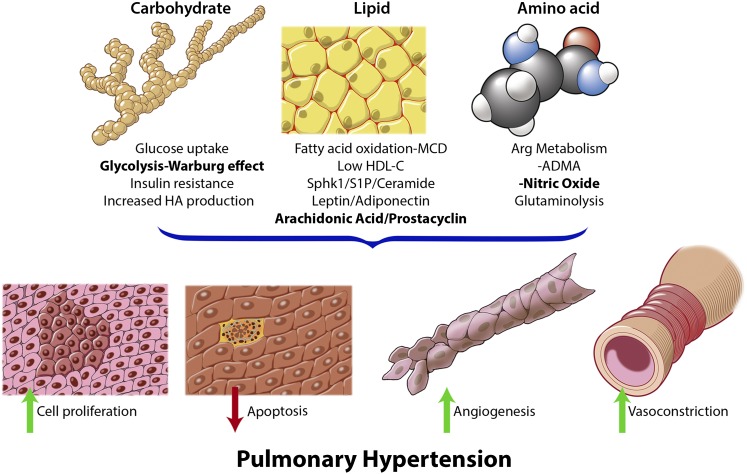
Pulmonary hypertension (PH) is a heterogeneous disorder likely to be composed of overlapping syndromes with varying origins and heterogeneous pathobiology and presenting with many phenotypes (1). Knowledge of the underlying pathobiology is necessary for understanding clinical disease manifestations and for devising specific and effective therapies. Enhanced pulmonary vascular cell proliferation, dysregulated cell apoptosis, increased angiogenesis, and vasoconstriction are hallmarks of the disease (Figure 1) (2) and lead to structural and morphological changes within the lung vasculature, including vascular remodeling and arterial wall narrowing. These dysfunctional processes lead to a progressive increase in pulmonary vascular resistance and, ultimately, right ventricular failure and death (3). At the molecular level, genetic factors and derangements in signaling pathways, cytokines, chemokines, and growth factors have been linked to the pathobiology of PH (4).
Figure 1.
Select identified metabolic derangements in pulmonary hypertension (PH). Dysregulated carbohydrate, lipid, and amino acid metabolism impacts the central phenotypic features of PH. On alteration, these derangements govern the changes in cell proliferation, apoptosis, angiogenesis, and vasoconstriction. These same metabolic derangements have been documented in cancer. At this time, several metabolic pathways are targets for PH therapy (bold). ADMA = asymmetric dimethylarginine; Arg = arginine; HA = hyaluronan; HDL-C = high-density lipoprotein cholesterol; MCD = malonyl-CoA decarboxylase.
More recently, metabolic dysregulation has emerged as a major area of research in the pathobiology of PH (Figure 1). Just like cancer, PH is characterized by cell proliferation, apoptotic resistance, and increased angiogenesis (5). Also much like patients with cancer, patients with PH exhibit excessive cellular glucose uptake and increased glycolytic metabolism compared with healthy individuals (6, 7). Patients with PH also exhibit alterations in levels of leptin, adiponectin, high-density lipoprotein cholesterol, and insulin resistance (8–11). Cancer cells exposed to these metabolic alterations reprogram their metabolism and protein homeostasis to adapt to nutrient stress conditions, as well as to establish tumor development, progression, and survival. Often, these cells undergo changes in glycosylation (i.e., O-linked N-acetylglucosamine modification of proteins and hyaluronan production) and lipid metabolism (12–14). These observed metabolic changes in cancer are increasingly becoming recognized in the pathogenesis of PH as well (15, 16).
One such example is dysregulation in sphingosine 1-phosphate (S1P) metabolism, which is increasingly recognized for its direct involvement in cell proliferation. Two lipid kinases, sphingosine kinase (SphK) 1 and 2, catalyze the conversion of the sphingolipid, sphingosine, to S1P. SphK1 has been linked to several signaling pathways involved in cancer cell proliferation and survival (17). Overexpression of SphK1 has been observed in many tumor tissues, which results in the accumulation of S1P, increased cell proliferation, apoptotic resistance, and disease development and progression (18). Conversely, a reduction in SphK1 activity and subsequent S1P levels is associated with increased cellular ceramide levels, which have been linked to apoptosis and cell cycle arrest (18). Indeed, the homeostatic balance between ceramide and S1P levels (the ceramide/S1P rheostat) is a gauge for cell death or survival. A recent report made a possible link between SphKs and PH (19).
In this issue of the Journal, Chen and colleagues (pp. 1032–1043) examined the SphK1/S1P pathway in PH and show that it promotes pulmonary arterial smooth muscle cell (PASMC) proliferation (20). This finding was identified using a combination of rodent models of hypoxia-mediated PH, human explanted lungs, and isolated human PASMCs. By investigating the functional consequences of altering SphKs or S1P in PH, the authors show that overexpression of SphK1 or S1P stimulation promoted PASMC proliferation, whereas loss of SphK1 blocked the PASMC proliferation in PH. In human PH lungs and PASMCs, SphK1 (but not SphK2) was upregulated, which was consistent with their findings of increased S1P levels. In addition, the protective effect of SphK1 deficiency on hypoxia-induced PH was highlighted. These findings of the direct involvement of the SphK signaling pathway in PH vascular proliferation demonstrate the homeostatic balance that is required for sphingosine metabolism in PH. The molecular changes in the SphK1/S1P metabolic pathway, guided by the current knowledge of its role in cancer cell proliferation, may open new avenues to identify its role as a contributor to pulmonary vascular remodeling and a potential therapeutic target in PH.
In spite of the novel findings in this report, many questions remain unanswered. Are the metabolic features described here universal in PH? Or do they represent a novel phenotype? Because there were only a few samples from patients with PH analyzed in this study, it will be interesting to determine whether the same observations of increased SphK1 activity and S1P levels holds true in a larger population of patients with PH. What are the main contributors to the increased SphK1 and S1P levels (i.e., hypoxia, altered glucose, lipid, or protein metabolism)? Will targeting the SphK1 activity result in a reduction or reversal of the pulmonary vascular proliferation? Alternatively, could the direct delivery of exogenous ceramide or stimulation of ceramide synthesis slow or reverse the process? Addressing these questions will be necessary to determine the fate of this molecular pathway as a novel therapeutic target in PH. In the meantime, however, the findings by Chen and colleagues invite us to explore deeper the global effects of altered metabolism in the pathogenesis of PH, a journey that will hopefully open doors to new therapeutic targets in this deadly disease (3).
Footnotes
Supported by the Ruth L. Kirschstein F32 postdoctoral fellowship (F32HL120629 to J.B.) and the Programs of Excellence in Glycosciences (1P01HL10714 to R.A.D.), both from NHLBI.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, Hill NS, Humbert M, Kawut SM, Krowka M, et al. ATS Committee on Pulmonary Hypertension Phenotypes. An official American Thoracic Society Statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med. 2014;189:345–355. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrell NW, Archer SL, Defelice A, Evans S, Fiszman M, Martin T, Saulnier M, Rabinovitch M, Schermuly R, Stewart D, et al. Anticipated classes of new medications and molecular targets for pulmonary arterial hypertension. Pulm Circ. 2013;3:226–244. doi: 10.4103/2045-8932.109940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:365–369. doi: 10.1164/rccm.201209-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pullamsetti SS, Schermuly R, Ghofrani A, Weissmann N, Grimminger F, Seeger W. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:394–400. doi: 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- 5.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev. 2013;22:543–551. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, et al. Lung 18F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aytekin M, Tonelli AR, Farver CF, Feldstein AE, Dweik RA. Leptin deficiency recapitulates the histological features of pulmonary arterial hypertension in mice. Int J Clin Exp Pathol. 2014;7:1935–1946. [PMC free article] [PubMed] [Google Scholar]
- 9.Heresi GA, Aytekin M, Newman J, DiDonato J, Dweik RA. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:661–668. doi: 10.1164/rccm.201001-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 11.Santos M, Reis A, Gonçalves F, Ferreira-Pinto MJ, Cabral S, Torres S, Leite-Moreira AF, Henriques-Coelho T. Adiponectin levels are elevated in patients with pulmonary arterial hypertension. Clin Cardiol. 2014;37:21–25. doi: 10.1002/clc.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z, Vosseller K. O-GlcNAc in cancer biology. Amino Acids. 2013;45:719–733. doi: 10.1007/s00726-013-1543-8. [DOI] [PubMed] [Google Scholar]
- 13.Kultti A, Zhao C, Singha NC, Zimmerman S, Osgood RJ, Symons R, Jiang P, Li X, Thompson CB, Infante JR, et al. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. Biomed Res Int. 2014;2014:817613. doi: 10.1155/2014/817613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012;3:167–174. doi: 10.4331/wjbc.v3.i8.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L789–L799. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, Michelakis ED. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med. 2010;2:44ra58. doi: 10.1126/scitranslmed.3001327. [DOI] [PubMed] [Google Scholar]
- 17.Heffernan-Stroud LA, Obeid LM. Sphingosine kinase 1 in cancer. Adv Cancer Res. 2013;117:201–235. doi: 10.1016/B978-0-12-394274-6.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plano D, Amin S, Sharma AK. Importance of sphingosine kinase (SphK) as a target in developing cancer therapeutics and recent developments in the synthesis of novel SphK inhibitors. J Med Chem. 2014;57:5509–5524. doi: 10.1021/jm4011687. [DOI] [PubMed] [Google Scholar]
- 19.Byun HS, Pyne S, Macritchie N, Pyne NJ, Bittman R. Novel sphingosine-containing analogues selectively inhibit sphingosine kinase (SK) isozymes, induce SK1 proteasomal degradation and reduce DNA synthesis in human pulmonary arterial smooth muscle cells. Medchemcomm. 2014;4 doi: 10.1039/C3MD00201B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:1032–1043. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]