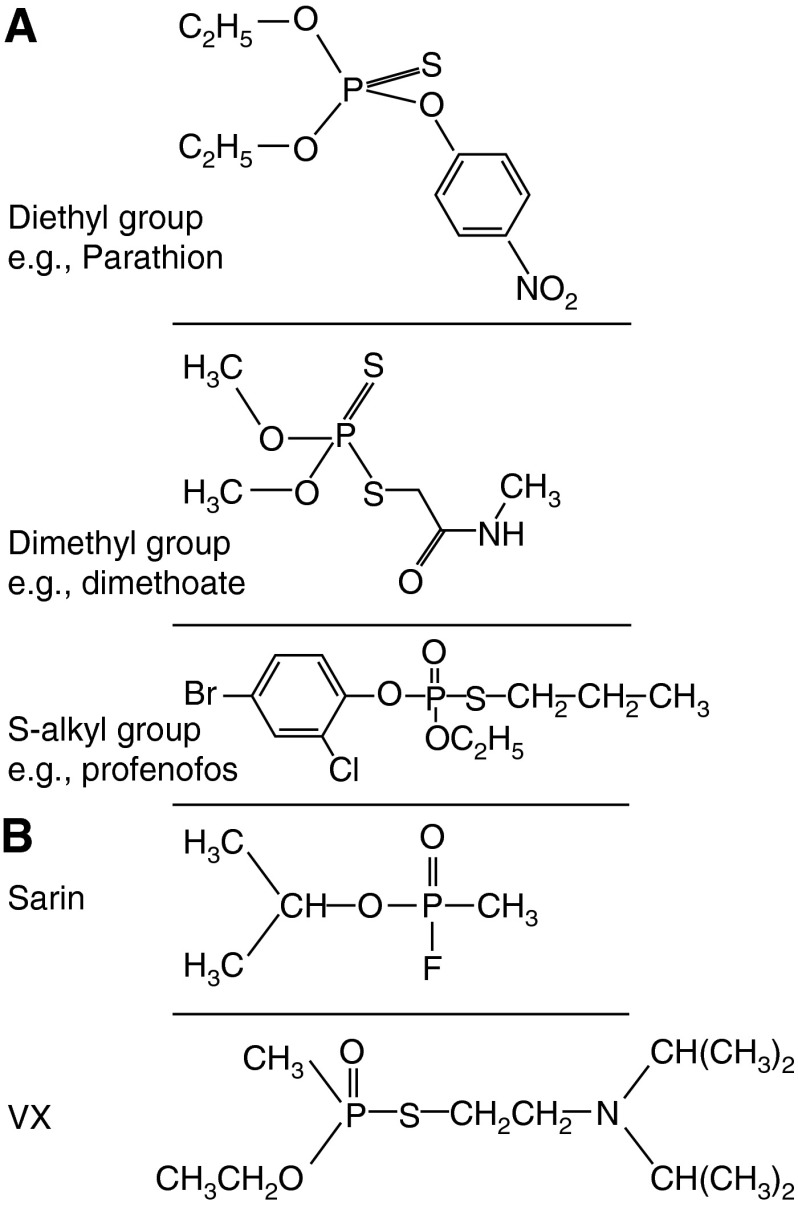
Figure 1.
Structures of organophosphorus (OP) insecticides and chemical weapons. (A) Structures of the diethyl, dimethyl, and S-alkyl OP insecticides parathion, dimethoate, and profenofos, respectively. The great majority of insecticides are either dimethyl or diethyl; inhibition of acetylcholinesterase produces a diethylated or dimethylated phosphate atom that does not vary according to the individual OP involved. Both parathion and dimethoate are “thion” pro-poisons that require activation by cytochrome P450s to active “oxons” that have the P = O group. Profenofos exists as an oxon and does not require activation. (B) Structures of the OP nerve agents sarin and VX.