Figure 3.
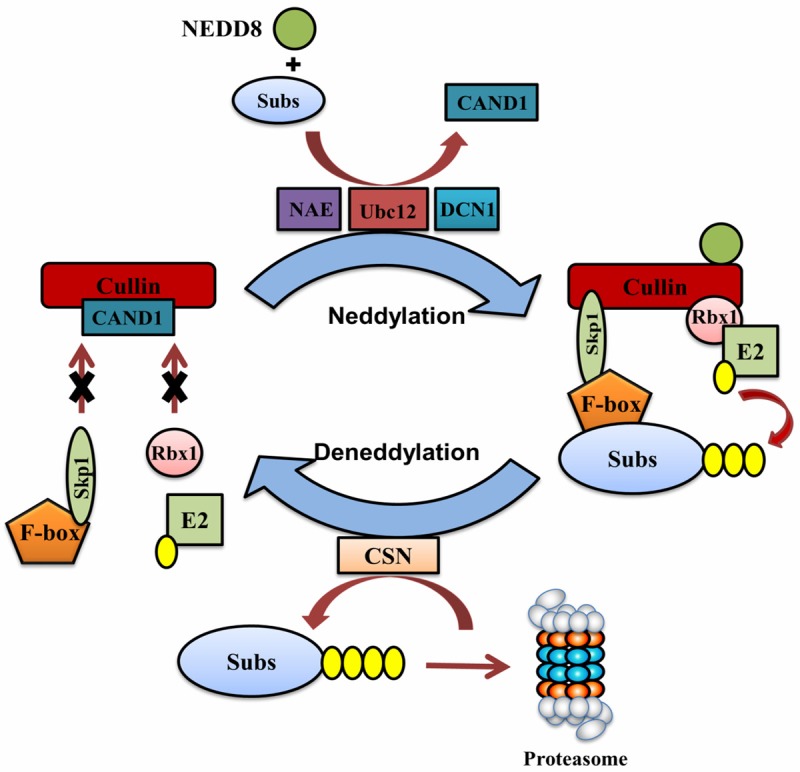
Regulation of cullin-RING ubiquitin ligase (CRL) activity by neddylation and deneddylation. CRL consists of a scaffold protein cullin, a RING protein Rbx1 that recruits ubiquitin E2, an adaptor protein Skp1 that interacts with a F-box protein, and a substrate (Subs)-recognizing F-box protein. NAE-Ubc12-DCN1-mediated neddylation of cullin changes the conformation of cullin, which prevents the binding of a CRL inhibitory protein CAND1 to cullin but allows the recruitment of Skp1 and Rbx1. The assembly of a functional CRL brings ubiquitin charged E2 and the substrate to the proximity, allowing the transfer of ubiquitin to the substrate. After ubiquitination, the deneddylase CSN removes NEDD8 from cullin, leading to the disassembly of CRL. The released cullin is then ready to recruit another ubiquitin charged E2 for next round of ubiquitination. Dynamic cycling of neddylation and deneddylation is essential for optimal CRL activity.
