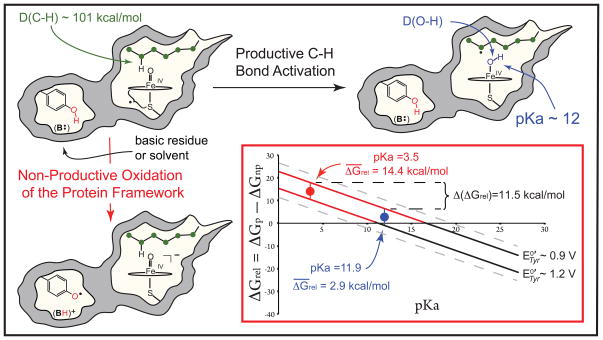
Fig. 1.
Productive and non-productive pathways for compound I decay. During productive C-H bond activation, compound I (a ferryl-radical species) abstracts hydrogen from substrate to yield compound II (an iron(IV)hydroxide complex) and a substrate radical. The non-productive pathway shown involves the oxidation of a tyrosine residue contained within the protein framework, with the phenolic proton being transferred to B:, a basic residue or solvent molecule, upon tyrosine oxidation. The inset shows the relative free energies (ΔGrel, Eq. 5) for the productive and non-productive pathways as a function of the compound II pKa for the range of aqueous tyrosine potentials. When ΔGrel is negative, the productive pathway is thermodynamically preferred. As a result of the ± 2 kcal/mol error in Eq. 2, a small range of tyrosine potentials could yield a given ΔGrel. The uncertainty in the solid lines as a result of this error is ± 0.1 V.