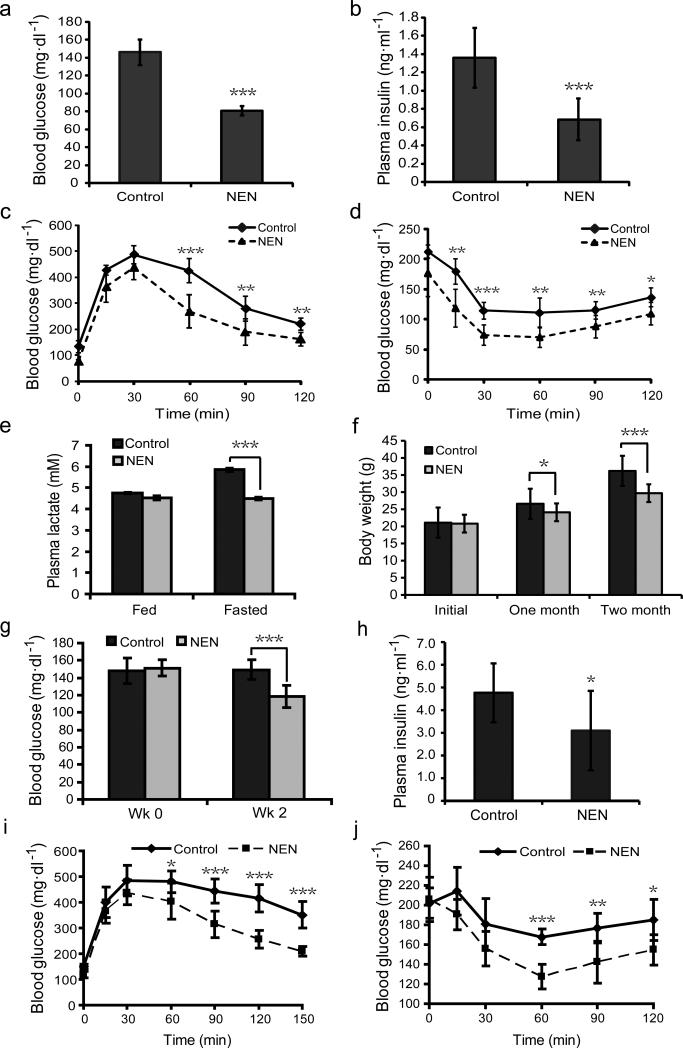
Figure 2.
Oral NEN is effective in preventing and treating HFD-induced insulin resistance. (a) Fasting blood glucose concentration of male C57BL/6 mice fed with either HFD (Control) or HFD with 1,500 ppm NEN (NEN), measured at wk 8 on HFD diet. (b) Basal plasma insulin concentration of the mice, as defined in a, measured at wk 8. (c) Glucose tolerance assay, and (d) insulin sensitivity assay of the mice, as defined in a, performed at wk 10. (e) Plasma lactate concentration of the mice, as defined in a, measured at wk 16 under indicated conditions. (f) Body weight of the mice, as defined in a, measured at indicated time points. (g) Fasting blood glucose concentration of male C57BL/6J mice that were first fed with HFD for 16 wk, and then randomized into two groups fed either with HFD (Control), or HFD containing 1,500 ppm NEN (NEN). The time when NEN containing food was initiated is designated as wk 0. (h) Basal plasma insulin concentration of the mice, as defined in g, measured at wk 2. (i) Glucose tolerance assay, and (j) insulin sensitivity assay of the mice, as defined in g, performed at wk 3. n = 7 for all groups. Student t-test, *P < 0.05; **P < 0.01; ***P < 0.001; error bar, s.d.