Fig. 4.6.
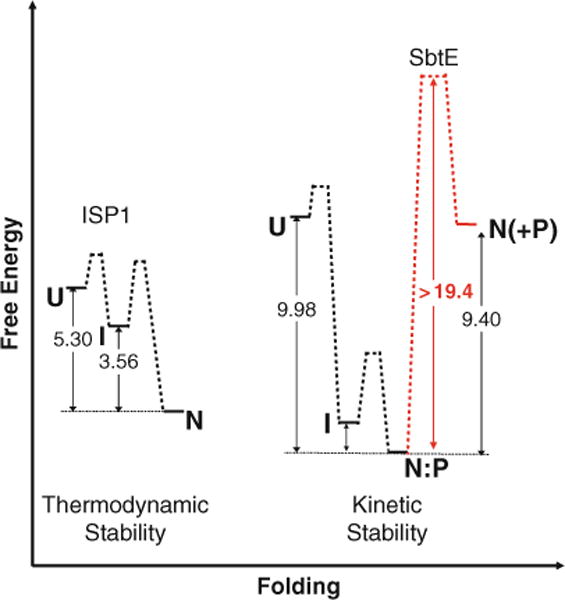
The free energy diagram depicting thermodynamic stability of ISPs and kinetic stability of ESPs – Unfolded ISP1 (U) spontaneously adopts its thermodynamically stable native state (N) through a partially folded intermediate (I). The free energy difference between N and U is approximately 5.3 kcal/mol in the case of ISP1 and is lowest compared to all experimentally observed states (111). In case of ESPs, the unfolded IMC-subtilisin (U) undergoes rapid folding and autoproteolysis to give a thermodynamically stable IMC:SbtE complex (N:P) through an intermediate state (I) (77). The structure of the IMC:SbtE complex has been solved using X-ray crystallography (76). The activation energy for the spontaneous release of the IMC from this complex is energetically unfavorable (approximately 21 kcal/mol and is shown in a broken light line). The release of the first free protease molecule is a stochastic process (110) and the subsequent steps occur by trans-proteolysis. Once folded, the high activation energy barrier kinetically traps folded SbtE in its native state. The free energy difference between the N:P and N is approximately 9.4 kcal/mol of energy. The net free energy difference between the unfolded and folded SbtE is ~0.4 kcal/mol and is likely to preclude spontaneous folding in the absence of the IMC.
