Figure 1. Inflammatory profiles in Alzheimers's disease.
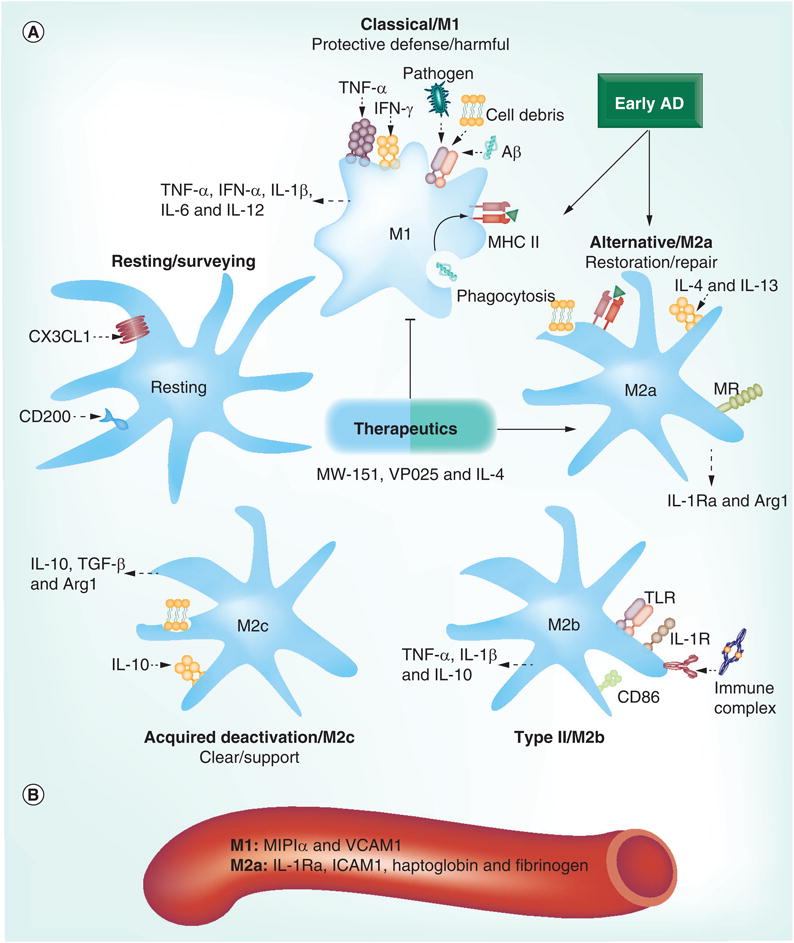
(A) Microglia activation states fall within a dynamic range that includes resting, M1, M2a, M2b and M2c. Resting microglia are kept quiescent by CD200 and CX3CL1 (fractalkine). Samples from patients with early AD indicate that their immune profile is polarized toward either an M1 or an M2a phenotype. Microglia become reactive to pathogens, neuronal debris and Aβ by activation of pattern-recognition receptors, including TLRs, which phagocytose these materials and present antigens with MHC II. Induction by and release of various pro- and anti-inflammatory cytokines differs across activation states and, taken together, can be used to identify these states. Therapeutics aimed at polarizing toward specific activation states, rather than robustly suppressing microglia activity, show promise for AD treatment. (B) Proteins from human blood (MIP1α and VCAM1 for M1; and IL-1Ra, ICAM1, haptoglobin and fibrinogen for M2a) may be useful peripheral markers to predict the central immune profile. Aβ: β-amyloid; AD: Alzheimer's disease; MR: Mannose receptor; TLR: Toll-like receptor.
