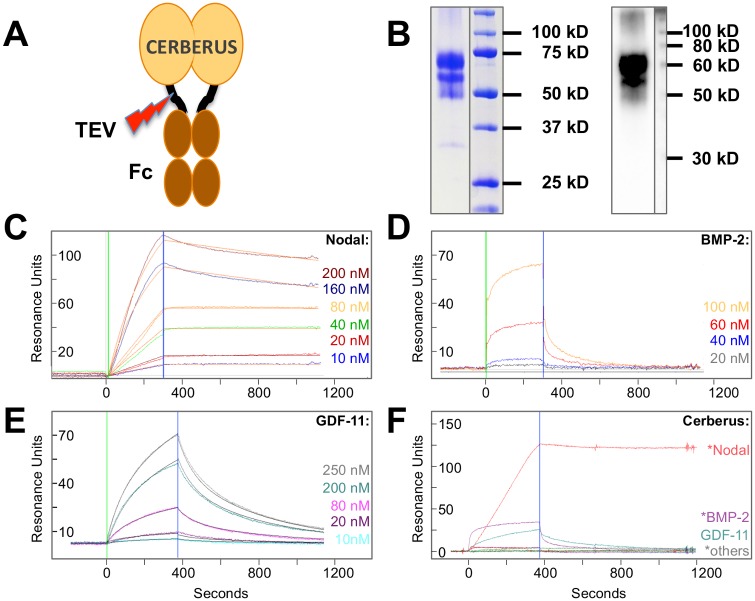
Figure 3. Cerberus ligand binding.
(A) Recombinant Cerberus construct. Full length human Cerberus was fused at the C-terminus to a human Igg1 Fc fragment via a linker containing a TEV cleavage site. (B) Coomassie Blue stained SDS-PAGE of Cerberus shown on the left side of the panel, Western blot using anti-Cerberus antibody RnD Systems, AF1075 is shown on the right side of the panel. Recombinant Cerberus is purified from Chinese Hamster Ovary cell conditioned medium using protein A capture. Overall, Cerberus-Fc is pure; size heterogeneity may be introduced by variations in glycan structure. The observed smaller Cerberus-Fc fragment could correspond is likely a proteolytic product. We have tested a Cerberus construct that is smaller than the proteolytic product (manuscript in preparation). Nodal binding activity of the shorter Cerberus-Fc is indistinguishable from full-length Cerberus-Fc. (C) Nodal-Cerberus interaction. Cerberus-Fc was immobilized on an SPR sensor chip and different concentrations of Nodal were injected as shown. The Nodal-Cerberus association constant (ka) is 1.3×104 M-1s-1, the dissociation constant (kd) is 1.4×10-5 s-1, and the equilibrium dissociation constant (Kd) is 1.0 nM. Fitted curves (orange lines) are superimposed over experimental curves. (D) BMP-2-Cerberus interaction. Cerberus-Fc was immobilized on an SPR sensor chip and different concentrations of BMP-2 were injected as shown. The sensograms could not be fitted to a global kinetic model due to the extremely fast dissociation rate. Single curve fitting and averaging yielded an estimated BMP-2-Cerberus association rate constant (ka) of ~2.4×104 M-1s-1, a dissociation constant (kd) of ~0.072 s-1, and an equilibrium dissociation constant (Kd) of ~3,000 nM. (E) GDF-11-Cerberus interaction. Cerberus-Fc was immobilized on an SPR sensor chip and different concentrations of GDF-11 were injected as shown. The sensograms were fit to a global kinetic model that yielded an association rate constant of 1.2×103 M-1s-1, a dissociation rate constant of 0.014 s-1, and an equilibrium dissociation constant of 5,800 nM. Fitted curves (grey lines) are superimposed over experimental curves. (F) Comparison of ligand binding to human Cerberus. Cerberus-Fc was immobilized on an SPR sensor chip and different TGFß family ligands were injected at a concentration of 80 nM as shown. Injections were performed at 40 µl/min. Ligands marked with an asterisk (*), including Nodal, BMP2 and others (Activin A and BMP4), have been shown to interact with Cerberus of different species. Nodal (red) most convincingly binds human Cerberus.