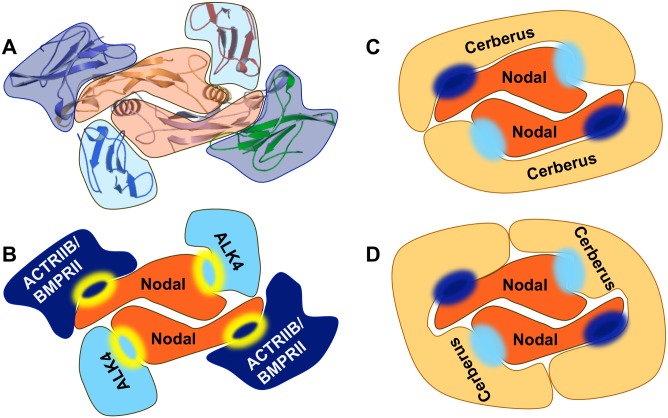
Figure 9. Proposed mechanism of Cerberus inhibition.
(A) Structure of BMP9-ALK1-ACTRIIB complex [37]. The disulfide linked BMP9 homodimer (center, orange) simultaneously binds the extracellular domains of the type I Activin receptor-like kinase ALK1 (light blue) and the type II Activin receptor kinase ACTRIIB (dark blue). (B) Molecular model of Nodal-receptor interactions based on the BMP9-ALK1-ACTRIIB structure. The disulfide linked Nodal homodimer (center, orange) binds the extracellular domains of the type I Activin receptor-like kinase ALK4 (light blue) and the type II Activin receptor kinase ACTRIIB or BMPRII (dark blue), likely using canonical interaction surfaces (yellow lined circles). (C-D) Cerberus forms a stable homodimer in solution [58], binds Nodal and prevents binding of Nodal to both receptors. Thus Cerberus blocks simultaneously the ALK4 interaction surface (light blue circle) and the ACTRIIB interaction surface (dark blue circle). We propose that one Cerberus protomer could block interaction surfaces within one Nodal protomer (C) or two protomers (D). We expect the overall stoichiometry of this complex to be 2:2.