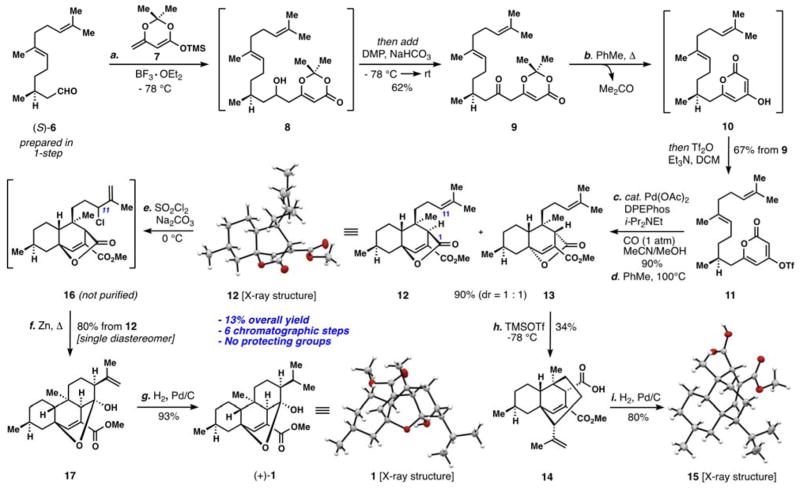
Scheme 1.
Enantioselective total synthesis of (+)-chatancin (1). Reagents and Conditions: a) 6 (1.0 equiv), 7 (1.1 equiv), BF3•OEt2 (1.5 equiv), CH2Cl2, −78 °C, 1 h, then add DMP (3.0 equiv), NaHCO3 (6.0 equiv), −78 °C → rt, 9 h, 62%; b) 9 (1.0 equiv) added slowly to PhMe, 120 °C → rt, 1.5 h, then Tf2O (1.0 equiv), Et3N (2.5 equiv), DCM, −78 °C → rt, 67% from 9; c) 11 (1.0 equiv), Pd(OAc)2 (10 mol %), DPEPhos (10 mol %), i-Pr2NEt (2.0 equiv), CO (1 atm), 4.2:1 MeCN/MeOH (v/v), rt, 8 h, 90%; d) PhMe (0.05 M), 100 °C, 4 d, 90% (12:13 = 1:1); e) 12 (1.0 equiv), SO2Cl2 (1.1 equiv), Na2CO3 (4.0 equiv), DCM, 0 °C, 30 min; Zn (40.0 equiv), THF, 65 °C, 24 h, 80% from 12; g) 17 (1.0 equiv), 5% Pd/C (10 mol %), H2 (1 atm), MeOH, 8 h, 93%; h) 13 (1.0 equiv), TMSOTf (2.0 equiv), DCM, −78 °C, 1 h, 34%; i) 14 (1.0 equiv), 5% Pd/C (10 mol %), H2 (1 atm), MeOH, 8 h, 80%. DMP = Dess Martin Periodinane, Tf = trifluoromethanesulfate, DPEPhos = Bis-[2-(diphenylphosphino)phenyl]ether.