Table 1.
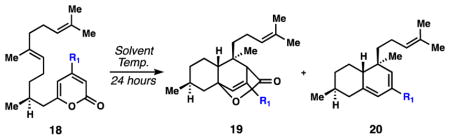
| ||||
|---|---|---|---|---|
| Entry | R1 | Solvent | Temp. (°C) | Ratio 18 : 19 : 20[b] |
| 1 | OH | toluene | 165 | 1 : 0 : 0 |
| 2 | OTf | toluene | 100 | 1 : 0 : 0.15 |
| 3 | CO2Me | heptane | 100 | 1 : 0.9 : 0.02 |
| 4 | CO2Me | PhCF3 | 100 | 1 : 1.4 : 0.07 |
| 5 | CO2Me | MeCN | 100 | 1 : 2.4 : 0.4 |
| 6 | CO2Me | DMF | 100 | 1 : 3.1 : 0.7 |
Conditions: 18 (0.03 M in solvent).
Ratios determined by 1H NMR. Cycloadducts (19) were formed as an approximate 1:1 mixture of diastereomers.
