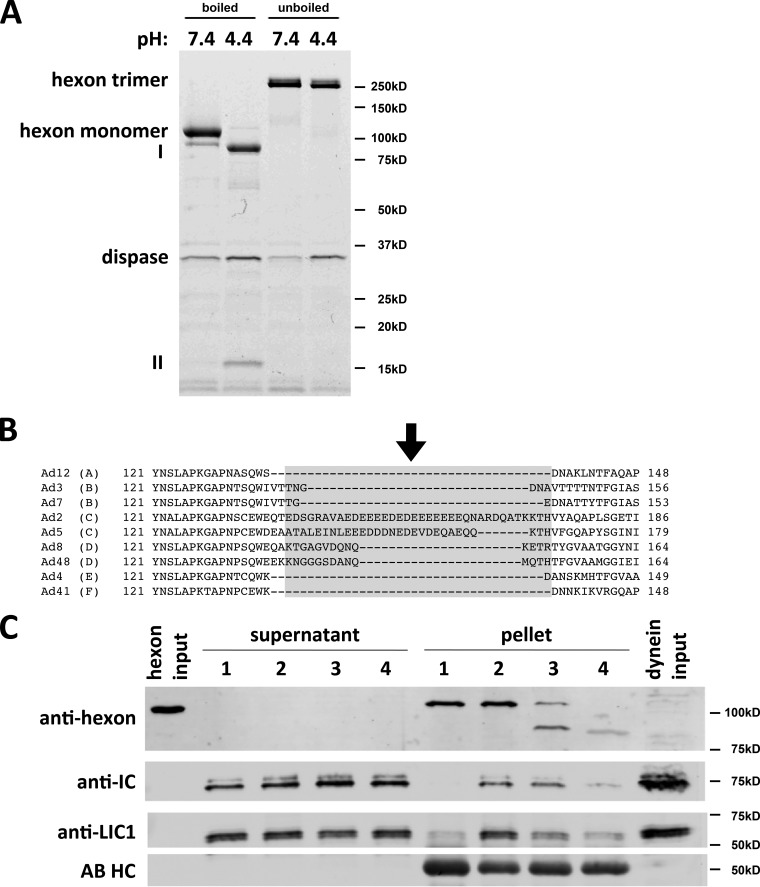
FIG 4.
Dispase hydrolysis inhibits low-pH effects on hexon structure and dynein binding. (A) Purified hexon was tested for dissociation after dispase treatment. Dispase exposure of hexon at low pH resulted in two major digestion products (85 kDa [I] and 15 kDa [II]) visible by Coomassie blue staining of SDS-PAGE gels of boiled samples. Unboiled samples show only hexon trimers under digested and undigested conditions, indicating that the hexon trimer remains intact even after complete dispase hydrolysis at low pH. (B) Sequence alignment of HVR1 of hexon from adenovirus subgroups A (serotype 12), B (serotypes 3 and 7), C (serotypes 2 and 5), D (serotypes 8 and 48), E (serotype 4), and F (serotype 41). HVR1 is marked in light gray (37), and the dispase cleavage site in AdV5 hexon is marked by an arrow. (C) Effects of dispase treatment on dynein binding. Hexon was treated with dispase at pH 4.4 and incubated with the purified dynein complex at pH 7.4. Lane 1, nonacidified hexon; lane 2, acidified hexon; lane 3, incompletely dispase-digested hexon; lane 4, fully dispase-digested hexon. Inputs as well as supernatants and pellets from hexon pulldowns were subjected to SDS-PAGE and Western blotting with antihexon, anti-LIC1, and anti-IC antibodies. Dispase pretreatment reduced the hexon affinity for the dynein complex. No proteolysis of dynein subunits was detectable. AB HC, antibody heavy chain.