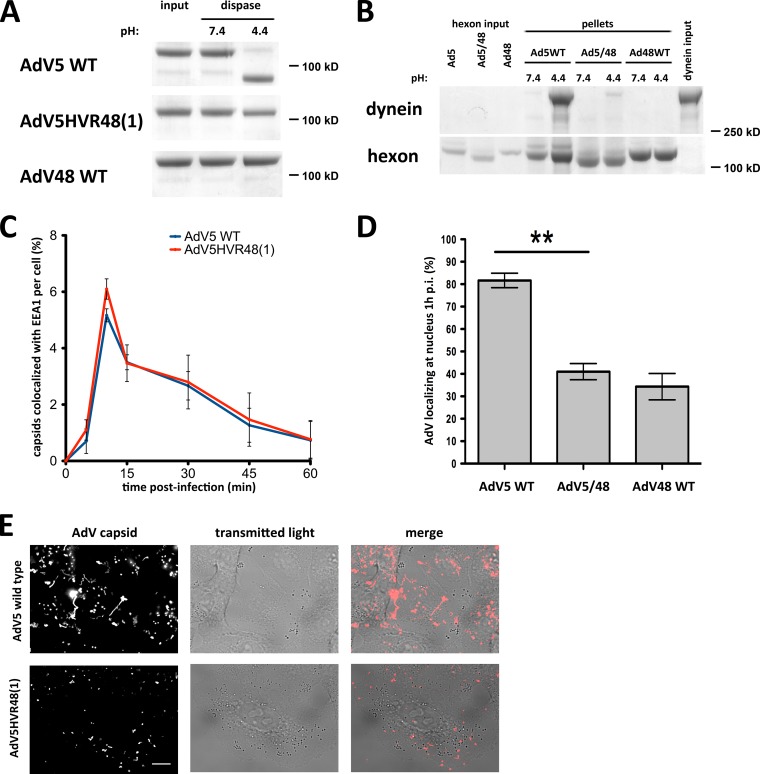
FIG 6.
The AdV5 hexon HVR1 mutant is inhibited in dynein binding and cytoplasmic transport. (A) Dispase insensitivity of mutant hexon. Immunopurified hexons of wild-type (WT) AdV5, AdV5HVR48(1), and WT AdV48 were tested for dissociation after dispase treatment. Dispase hydrolyzed only WT AdV5 hexon at low pH, visible by Coomassie blue staining of SDS-PAGE gels of boiled samples. AdV5HVR48(1) and WT AdV48 samples show hexon trimers independent of pH, indicating that the hexon trimer remains intact even after dispase hydrolysis and low-pH treatment. (B) Effect of mutant HVR1 on dynein binding. Immunopurified hexons from WT AdV5, AdV5HVR48(1) (AdV5/48), and WT AdV48 were exposed to pH 7.4 or pH 4.4 and tested for dynein binding at pH 7.4. Only acid-primed WT AdV5 hexon, but not acid-primed AdV5HVR48(1) or WT AdV48 hexon or hexon kept at pH 7.4, pulls down significant amounts of dynein, as shown by Coomassie blue staining of SDS-PAGE gels of boiled samples. (C) Lack of an effect of mutant HVR1 on virus entry. A549 cells were infected with WT AdV5 or AdV5HVR48(1) capsids, fixed at the indicated times p.i., and processed for immunofluorescence microscopy using antihexon and anti-EEA1 antibodies. The graph shows the percent colocalization of AdV capsids with EEA1 over time and indicates similar profiles for WT and mutant AdV5. (D) Effect of the altered HVR1 sequence on virus redistribution to the nucleus. AdV5HVR48(1) and WT AdV48 each show a significant decrease in nuclear localization at 1 h p.i. relative to WT AdV5 (n = 3; means ± standard deviations). Asterisks mark P values of <0.01. (E) Effect of mutant HVR1 on virus motility. A549 cells were infected with Alexa-labeled WT AdV5 or AdV5HVR48(1) capsids and immediately analyzed by live-cell microscopy (see Materials and Methods). Shown are time stack projections of capsids (AdV capsid) and individual transmitted light micrographs of representative movies for each AdV. WT AdV5 capsids show long-range motility, whereas AdV5HVR48(1) motility was restricted (see Movies S1 and S2 in the supplemental material). Bar, 10 μm.