FIG 6.
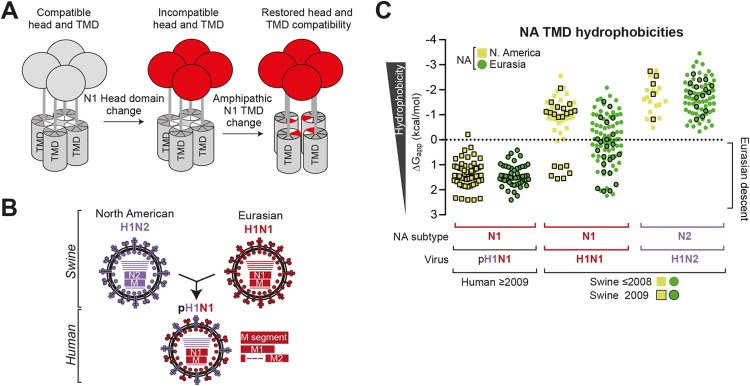
The Eurasian swine origin of the pN1 head domain can be traced by its low TMD hydrophobicity. (A) Model displaying how N1 head domain changes disrupt the folding compatibility with the N1 TMD. Head domain changes create selection pressure for residues (mainly polar) in the TMD that alter its amphipathic assembly and restore the compatibility. (B) Diagram showing the reassortment of the NA (N1 and N2) and M segments from geographically separated North American swine H1N2 viruses and the Eurasian swine H1N1 viruses that created the 2009 human pandemic pH1N1 virus. (C) Prior to 2009, the low N1 TMD hydrophobicity found in the pH1N1 viruses globally was present only in the swine H1N1 viruses from Eurasia (see “Swine ≤2008”). All the predicted N1 and N2 TMD hydrophobicities from the available swine (H1N1 and H1N2) and human pH1N1 viruses are displayed with respect to their geographic location and date of isolation (2009 and ≤2008).
