FIG 1.
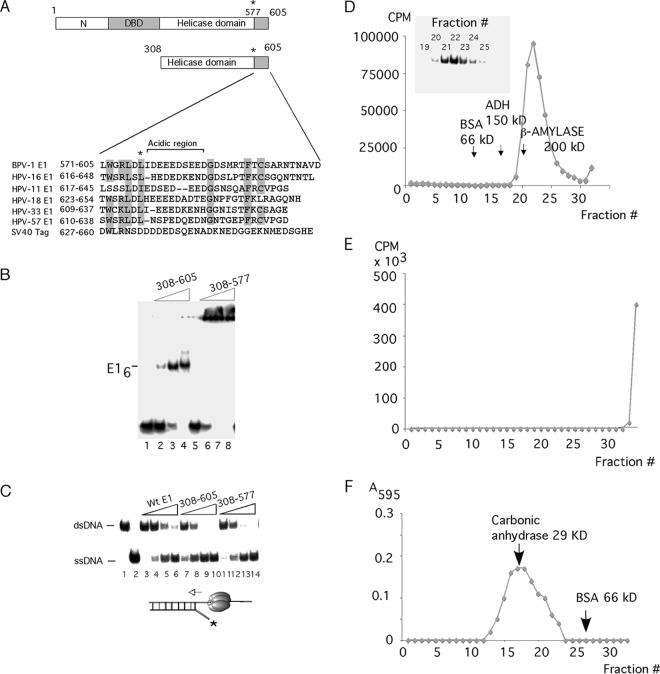
A C-terminal 28-aa peptide is important for complex formation by E1308–605. (A) The C-terminal regions from 6 different papillomavirus E1 proteins and simian virus 40 (SV40) T-ag were aligned. Highly conserved residues are highlighted, and the acidic region is bracketed. (B) A 41-mer ssDNA probe was incubated with three levels (8, 16, and 32 ng) of E1308–605 (lanes 2 to 4) or E1308–577 (lanes 6 to 8) in the presence of ADP and subjected to EMSA. In lanes 1 and 5, no protein was added. The position of the E1 hexamer complex is indicated. (C) Two femtomoles of helicase substrate was incubated with four quantities (8, 16, 32, and 64 ng) of full-length E11–605 (lanes 3 to 6), E1308–605 (lanes 7 to 10), and E1308–577 (lanes 11 to 14) in the presence of ATP. Lane 1 contained probe alone, while lane 2 contained boiled probe. The migrations of dsDNA and ssDNA are indicated. (D) Binding reactions for EMSA with E1308–605, as shown in panel B, were scaled up and sedimented in a 15 to 30% glycerol gradient. The radioactivity in the fractions was quantitated and plotted. The peak fractions were loaded onto an EMSA gel to verify that the gradient peak corresponded to the complex that we observe by EMSA (inset). Arrows indicate the sedimentation of the marker proteins bovine serum albumin (BSA), alcohol dehydrogenase (ADH), and β-amylase. (E) Binding reactions for EMSA with E1308–577, as shown in panel B, were scaled up and sedimented in a 15 to 30% glycerol gradient. The radioactivity in the fractions was quantitated and plotted. The last fraction plotted corresponds to the bottom portion of the tube, which was cut off and quantified. (F) One hundred micrograms of E1308–577 was sedimented in a 5 to 30% glycerol gradient. The protein was detected by Bradford assays and compared to the sedimentation of the marker proteins carbonic anhydrase and BSA.