Figure 2.
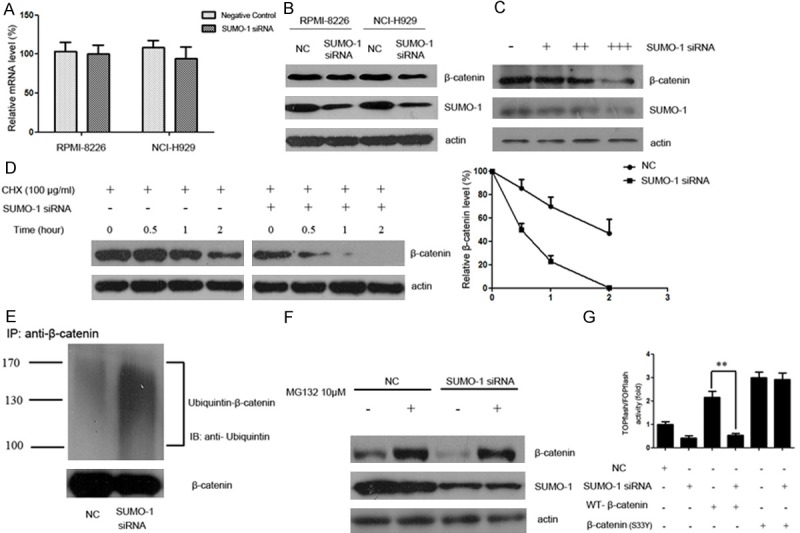
SUMOylation inhibition promotes the degradation of β-catenin via the ubiquitin-proteasomal pathway. A. Myeloma cells were treated with SUMO-1 siRNA or negative control for 48 hours. Total RNA was then extracted, and β-catenin mRNA level was determined by real time RT-PCR. B. Myeloma cells NCI-H929 and RPMI-8226 were transfected with control or siRNA targeting SUMO-1. After 48 h incubation, whole cell lysate was collected and lysed and immunoblotted with anti-SUMO-1 or anti-β-catenin antibody respectively. C. Myeloma cells NCI-H929 were treated with mounting doses of SUMO-1 siRNA (30 nM, 50 nM, 100 nM) for 48 h. Then whole cell lysate was collected and lysed and immunoblotted with anti-SUMO-1 or anti-β-catenin antibody respectively. D. Myeloma cells were transfected with control or siRNA targeting SUMO-1. After 48 hours, cells were treated with CHX (100 μg/ml) for indicated time periods (0, 0.5, 1, 2 hours). Cell lysate was subjected to western blot with anti-β-catenin antibody. The relative intensity of β-catenin proteins at the indicated time points was quantified using SmartViewsoftware. In the negative control group, the intensity was standardized to 100% of the control sample (CHX 0 h); while in the SUMO-1 siRNA group, it was standardized to 100% of the control sample in the SUMO-1 siRNA group (CHX 0 h). This result is a representative of three independent experiments. E. Myeloma cells NCI-H929 were transfected with SUMO-1 siRNA or negative control. After 48 hours, the cells were harvested under denaturing conditions and immunoprecipitated with anti-β-catenin antibody. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-ubiquitin and anti-β-catenin antibodies respectively. F. Myeloma cells NCI-H929 were transfected with SUMO-1 siRNA or negative control. 48 hours after transfection, cells were treated with MG132 (10 μM) for 5 hours. The protein extracts were then analyzed by western blot using antibodies against β-catenin, SUMO-1 or actin as loading control. G. Myeloma cells were transfected with SUMO-1 siRNA together with β-catenin (WT) or β-catenin (S33Y) plasmids. After 48 h incubation, cells were transfected with TOPflash reporter plasmid or FOPflash-negative control plasmid. Twenty-four hours after retransfection, cells were harvested and luciferase activity was measured as described in Materials and Methods. All results were normalized for transfection efficiency using the hRL-Null Renilla plasmid. Fold induction corresponds to luciferase activity of positive TOPflash reporter over negative FOPflash reporter. (Data represent mean ± S.D.; **p < 0.01 by Student’s t-test).
