Abstract
To determine the role of JAK-2/STAT-3 signaling pathway in invasion and vasculogenic mimicry of laryngeal squamous cell carcinoma. HEp-2 cells were treated with 1 or 10 μmol/L curcumin and AG490 (the inhibitor of JAK-2) for 48 h, the invasion and vasculogenic mimicry of tumor cells were tested with Transwell chamber test and tube formation experiment. RT-PCR was used to measure the expression of MMP-2 and VEGF. Western blot assay was employed to determine the expression of JAK-2, STAT3, p-STAT3, MMP-2 and VEGF. Compared to control group,there were less tumor cells permeating membrane and less formed tubes after curcumin or AG490 treatment, RT-PCR showed that the expression of MMP-2 and VEGF at mRNA level were decreased (P < 0.01). Western blotting indicated that the expression of JAK-2, p-STAT3, MMP-2 and VEGF at protein levels were decreased (P < 0.01), while that of STAT-3 protein had no difference among each group (P > 0.05). Immunofluorescence staining demonstrated that the expression of eNOS was down-regulated (P < 0.01). Curcumin and AG490 significantly inhibits invasion and vasculogenic mimicry of laryngeal squamous cell carcinoma in vitro, and JAK-2/STAT-3 signaling pathway promotes above processes.
Keywords: Laryngeal squamous cell carcinoma, curcumin, JAK-2/STAT-3 signaling pathway, vasculogenic mimicry
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common type of cancer worldwide, representing about 6% of all cancer cases [1]. Laryngeal squamous cell carcinoma (SCC) has the second highest incidence of all head and neck squamous cell carcinomas. In recent years, the incidence of laryngeal cancer is about 160,000 new cases diagnosed per year [2]. Despite significant advances in surgery and radiotherapy over the last few decades, no treatment has been shown to achieve a satisfactory therapeutic outcome and the mortality rate of laryngeal SCC is still high, with a 5-year survival rate of 64% [3]. Given the high mortality rate of laryngeal SCC, it is a critical need to explore the molecular pathogenesis and develop the new relevant biomarker to increase specificity or sensitivity for early diagnosis and prognosis.
In 1999, Maniotis [4] reported that blood vessels of highly aggressive uveal melanomas are formed by tumor cells instead of endothelial cells. He termed this novel concept in tumor vasculogenic mimicry (VM). The discovery of PAS-positive channels in the microcirculation of highly aggressive uveal melanomas initiated studies on VM. Light microscopy, transmission EM, and immunohistochemical staining reveal that PAS-positive pattern channels are lined externally by melanoma cells but have no inner lining of endothelial cells [4-7]. Since then, VM has been seen in several malignant tumor types such as breast cancer, lung cancer, kidney cancer, ovarian cancer, melanoma, and prostate cancer [8-13].
At present little is known about the molecular mechanisms involved in VM. It is therefore difficult to propose a precise clinical-pathological relationship and tumor therapy strategy. Many investigators involved in basic research on VM are trying to find an anti-VM therapy in laryngeal SCC [14]. Therapies targeting VM have only been performed in vitro till date [15]. Recently, STAT-3 was identified as important mediators of VM [16]. This study documented that the anti-VM effect of curcumin was due to inhibition of STAT-3 phosphorylation, as confirmed by specific inhibitors. Others have reported that PI3K is important for angiopoietin-1-mediated endothelial cell sprouting by regulating MMP-2 [17] critical for angiogenesis. On the basis of these observations, we sought to investigate the potential role of JAK-2/STAT-3 as a mediator of VM of squamous cell carcinoma of the larynx.
Curcumin, the major yellow coloring pigment found in the household spice turmeric, has been used for centuries in food preparation [18]. Curcumin has low toxicity and has been shown to have antineoplastic potential, inhibiting the development of chemically induced tumors of the oral cavity, skin, forestomach, duodenum and colon in rodents [19]. The effect of curcumin on pathological angiogenesis associated with laryngeal squamous cell carcinoma has not been defined. In this study, we tested the hypothesis that JAK-2 regulates VM in laryngeal SCC by mediating the activities of STAT-3. Addition of curcumin and AG490, a specific inhibitor of JAK-2, inhibited the ability of HEp-2 cells to engage in VM on 3-dimensional type-I collagen matrices and to invade a defined matrix in vitro. Furthermore, addition of this inhibitor decreased the levels of active JAK-2 and the expression of pSTAT-3 and the activity of MMP-2 in vitro. Moreover, Western blot analyses revealed a decrease in the levels of the VEGF after inhibition of STAT-3. Taken together, these results implicate JAK-2 as a key regulator of laryngeal squamous cell carcinoma VM by mediating the activation of STAT-3 which may serve as new molecular targets for therapeutic intervention of the signaling cascade underlying this unique process.
Material and methods
Cell culture and proliferation assay
HEp-2 cell line was originally thought to be derived from an epidermoid carcinoma of the larynx. In this study, HEp-2 cells were purchased from Clontech (San Diego, CA, USA) and were cultivated in DMEM low-glucose medium supplemented with 10% inactivated fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin under standard culture conditions (37°C, 95% humidified air and 5% CO2). Supernatant and cell lysates were collected at 3 days after reseeding. A total of 3 × 104 HEp-2 cells per well were seeded onto fibronectin-coated 24-well plates, and proliferation assays were performed according to the manufacturer’s instructions. After pretreatment with 1 μM/10 μM curcumin and 1 μM AG490 for 6, 12, 24 and 48 h, or left untreated. Then, cells were re-suspended and counted. Each condition was assessed in triplicate.
HEp-2 cell Transwell migration assay
The chemotactic motility of HEp-2 cells was determined using Transwell migration chambers (BD Biosciences) with 6.5-mm-diameter polycarbonate filters (8-μm pore size) as described previously [20]. In brief, cells were pretreated with curcumin or AG490 for 1-3 hours as indicated. Thereafter, the bottom chambers were filled with 600 μL of DMEM media containing all supplements. HEp-2 cells (3 × 104 per well) were seeded in top chambers in 100 μL DMEM media without serum. Cells were allowed to migrate for 8 h. Non-migrated cells were removed with cotton swabs, and migrated cells were fixed with ice cold ethanol and stained with 0.01% crystal violet. Images were captured using Olympus DP72 digital camera on Olympus microscope with magnification x100 and invasive cells were quantified by manual counting.
HEp-2 cell capillary-like tube formation assay
To examine the effect of curcumin on in vitro angiogenesis, tube formation assay was performed as described previously [21]. Matrigel-Matrix (BD Biosciences, Franklin Lakes, New Jersey, USA) was pipetted into pre-chilled 96-well plates (50 μL matrigel per well) and polymerized for 45 min at 37°C. HEp-2 cell (2 × 104 per well) in complete media were simultaneously seeded in matrigel coated plates. Then culture plates were exposed to curcumin/AG490 for 1-3 hours as indicated. After 12 h of incubation, tubular structures were photographed. Images were acquired under a fluorescent microscope (IX-71; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color cooled camera (DP72; Olympus). Capillary tube branch points were counted in six randomly selected fields per well, and used as an index for tube formation.
Western blot analysis
For in vitro protein analysis HEp-2 cell samples were homogenized and processed for Western Blotting according to the manufacturer’s instructions. HEp-2 cells were processed for western blotting as suggested by the manufacturer. Total JAK-2, phospho-STAT-3 (p-STAT-3), MMP-2 antibodies were purchased from Santa Cruz (California, USA). Polyclonal anti-VEGF anti-body was purchased from Abcam (Cambridge, UK). GAPDH antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
Immunofluorescence staining
HEp-2 cell monolayers were grown on coverslips to 80% confluence. Following curcumin or AG490 treatment, monolayers were rinsed once in phosphate-buffered saline (PBS), fixed with cold methanol for 30 min and blocked with 5% bovine serum albumin in PBS with Ca2+ and Mg2+ for 60 min. Using eNOS antibody, followed by a secondary antibody (Invitrogen, Carlsbad, CA, USA), the effect of curcumin on eNOS expression was visualized. Coverslips were mounted on Superfrost slides (Fisher Scientific) with Prolong Antifade mounting medium (Invitrogen) and visualized using a fluorescence microscope (Olympus BX-40) and a Leica DFC 300FX camera. Analyses were performed by a single blinded researcher.
RNA preparation and semi-quantitative reverse transcription-PCR (RT-PCR)
Total RNA isolation from samples was performed using the TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. The concentrations and quality of the RNA were determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, DE USA) and agarose gel electrophoresis. The expression of VEGF and MMP-2 mRNAs was assayed using TaqMan mRNA reverse transcription assays (Applied Biosystems) and appropriate primers (Applied Biosystems) following the manufacturer’s instructions. The primers for VEGF were using forward (5’-GAGGGCAGAATCATCACGAA-3’) and reverse (5’-GGGAACGCTCCAGGACTTAT-3’); MMP-2 forward (5’-TTGCTGCCACAAGAACTG-3’) and reverse (5’-TTGAAAGACTGGGAGAAG-3’); GAPDH forward (5’-GGGAAACTGTGGCGTGAT-3’) and reverse (5’-AAAGGTGGAGGAGTGGGT-3’), respectively. The reaction conditions indicated in the manufacturer’s manual were used and the reaction mixtures were incubated at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The ΔΔCt method for relative quantization was used to determine mRNA expression levels. The ΔCt value was calculated by subtracting the Ct of GAPDH from the Ct of the mRNA of interest. The ΔΔCt value was calculated by subtracting the ΔCt of the reference sample from the ΔCt of each sample. The fold-change was determined as 2-ΔΔCt.
Statistical analysis
Data are expressed as means ± standard deviation (SD). One-way ANOVA was used for statistical analyses. SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA) was used. A value of p < 0.05 was considered significant.
Results
Curcumin inhibits HEp-2 cells growth, proliferation and migration
Vasculogenic mimicry involves multiple events in HEp-2 cells, including cell growth, proliferation and migration. To determine the anti-vasculogenic mimicry potential of curcumin and its potential mechanism of action through inhibition of JAK-2 expression, in vitro, vasculogenic mimicry assays measuring growth, proliferation, and transmigration were performed in HEp-2 cells, using AG490 as a specific JAK-2 inhibitor. Initial experiments were performed evaluating HEp-2 cells growth in a Transwell migration chambers, with cell expansion across a polycarbonate filters. Figure 1A demonstrates a potent anti-vasculogenic mimicry effect of 10 μM curcumin compared with control cells. 1 μM Curcumin-pretreated HEp-2 monolayer grew at rates almost similar to those of untreated cells. Curcumin demonstrated no cytotoxicity at the dosages used in this study. The JAK-2 inhibitor AG490 was a potent inhibitor of HEp-2 cell growth (Figure 1B). Pretreatment of HEp-2 cells with curcumin or the JAK-2 specific inhibitor AG490 resulted in inhibition of cell transmigration. 10 μM curcumin pretreatment of HEp-2 cells inhibited transmigration, which was similar to the effect of the JAK-2 specific inhibitor AG490 (Figure 1C and 1D).
Figure 1.
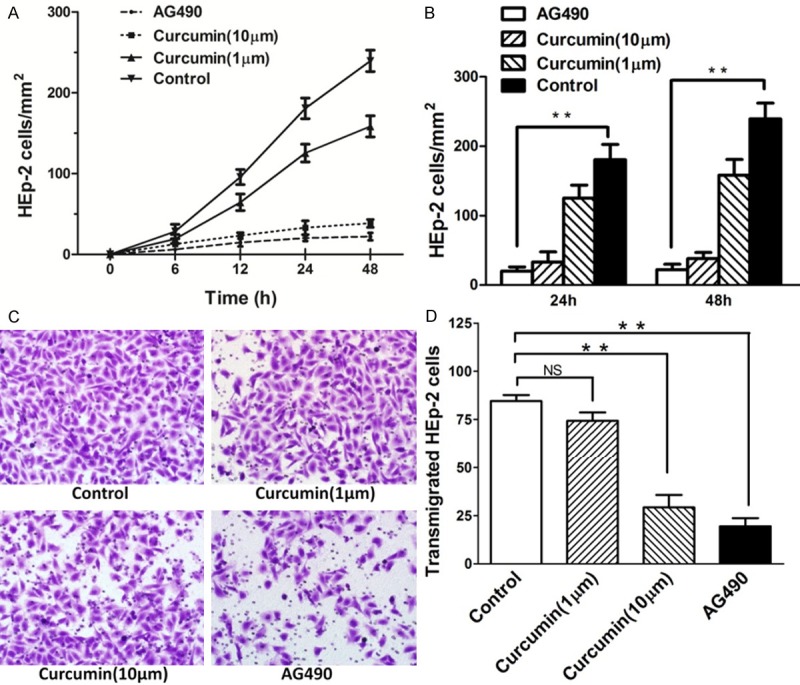
Curcumin inhibits growth, proliferation and migration in HEp-2 cells. A, B. 1 μM curcumin inhibited HEp-2 cells growth at rates almost similar to those of control cells. Potent anti-proliferation effect of 10 μM curcumin compared with no stimulation in HEp-2 cells. The JAK-2 inhibitor, AG490 was a potent inhibitor of cell growth. C. ffect of curcumin on the migratory potential of HEp-2 cells was examined using Transwell migration chambers. D. The number of HEp-2 cells transmigrated through the filter was decreased by curcumin stimulation; 10 M curcumin pretreatment of HEp-2 cells significantly inhibited HEp-2 cells transmigration. At least 15 random high-power fields (×200) per condition were counted and data were expressed as mean (SD). **P < 0.01, versus control group.
Curcumin regulates HEp-2 cells tube formation in vitro
To address the role of JAK-2/STAT-3 signaling pathway during laryngeal squamous cell carcinoma VM, a specific inhibitor to JAK2 was used, AG490. The laryngeal carcinoma cell line HEp-2 was cultured on a 3D-matrix (consisting of type I collagen) and left either untreated or treated with curcumin or AG490 for a period of 8 hours. The number of HEp-2 cells tubes formed in Matrigel was significantly inhibited by both curcumin and AG490 (Figure 2A). Within the range of 1-10 μM curcumin inhibited tube formation of the HEp-2 cells in a dose-dependent manner (Figure 2B). This inhibitory effect of curcumin was not due to its cytotoxicity because more than 95% cell viability was retained throughout the range of test concentrations. These results indicate that inhibition of JAK-2 activity is linked to impaired HEp-2 vasculogenic mimicry in vitro.
Figure 2.
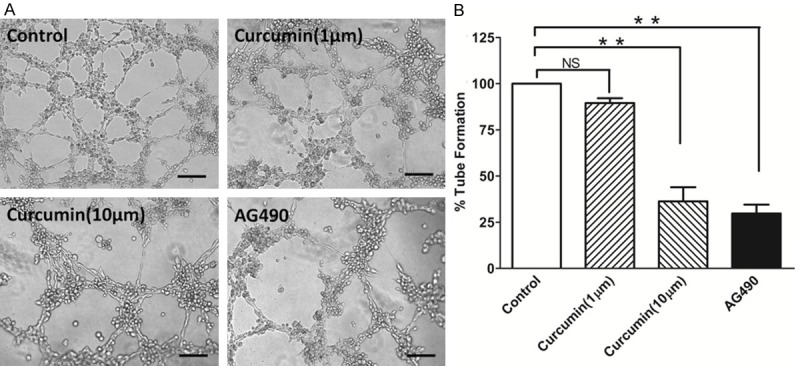
Effect of curcumin on tube formation of HEp-2 cells. The cells were seeded into Matrigel-coated wells and allowed to form tubular structures. Phase-contrast photomicrograph demonstrates the HEp-2 cells in vitro tube formation on Matrigel; the formation of capillary-like structures was inhibited by curcumin pretreatment. Total tube length of the tubular network was quantified from the photographs taken 5 h after seeding. Tubular structures were photographed at 100x magnification (A) and tube length was measured (B). Data are expressed as the mean ± SD of three independent experiments, and significant differences from the control are indicated by **p < 0.01.
Curcumin inhibits JAK-2 expression and pSTAT-3 production in HEp-2 cells
Next we examined the effect of curcumin on JAK-2 protein expression. Pretreatment of HEp-2 cells with curcumin inhibit JAK-2 protein expression in a dose-dependent fashion. Western blot analyses of whole cell lysates from these experimental samples confirmed a reduction in JAK-2 activity after addition of curcumin. The selective inhibitor of JAK-2, AG490 (1 μM), abolished JAK-2 protein expression in HEp-2 cells (Figure 3A, 3B). Corresponding with the effect on JAK-2 expression, curcumin inhibited pSTAT-3 production, but not STAT-3, in a dose-dependent fashion as determined by west-blot measurement of HEp-2 cells culture media. The pSTAT-3 production was completely inhibited by 1 μM AG490, indicating that production of pSTAT-3 is dependent on JAK-2 activity in HEp-2 cells (Figure 3C, 3D).
Figure 3.
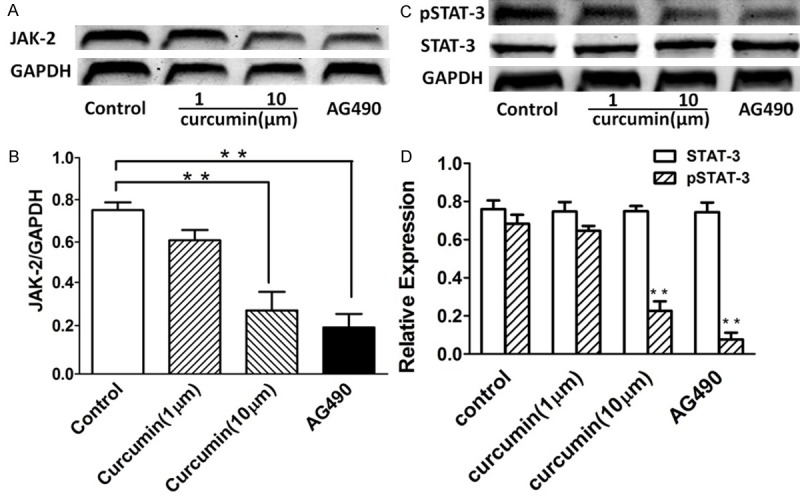
Effect of curcumin on JAK-2 expression and pSTAT-3 production in HEp-2 cells. We further investigated whether the anti-VM activity of curcumin is associated with suppression of the invasive phenotype of the cells. (http://gut.bmj.com/content/57/11/1509.full - F2). A, B. Curcumin pretreatment of HEp-2 cells inhibited JAK-2 protein expression at 1 μM, with increasing effect at 10 μM curcumin. When the effect of AG490 on JAK-2 production of HEp-2 cells was tested, a significant inhibition was observed at a concentration of 1 μM AG490. C, D. Curcumin inhibited pSTAT-3 production, but not STAT-3, in HEp-2 cells in a dose-dependent manner. Data were expressed as mean ± SD. **p < 0.01 compared with no stimulation.
Curcumin inhibits eNOS expression in HEp-2 cells
In addition to the effect of curcumin on JAK-2 expression, curcumin also affects expression of several genes associated with cell growth and/or apoptosis. Curcumin inhibition of endothelial nitric oxide synthase (eNOS) expression in endothelial cells has been shown to contribute to impaired endothelial tube formation [22]. In addition, immunofluorescence staining of HEp-2 cells pretreated with either 1 μM/10 μM curcumin or 1 μM AG490 demonstrated inhibition of eNOS expression (Figure 4). Taken together, our results suggest that the antiangiogenic activity of curcumin involves modulation of multiple pathways in HEp-2 cells.
Figure 4.
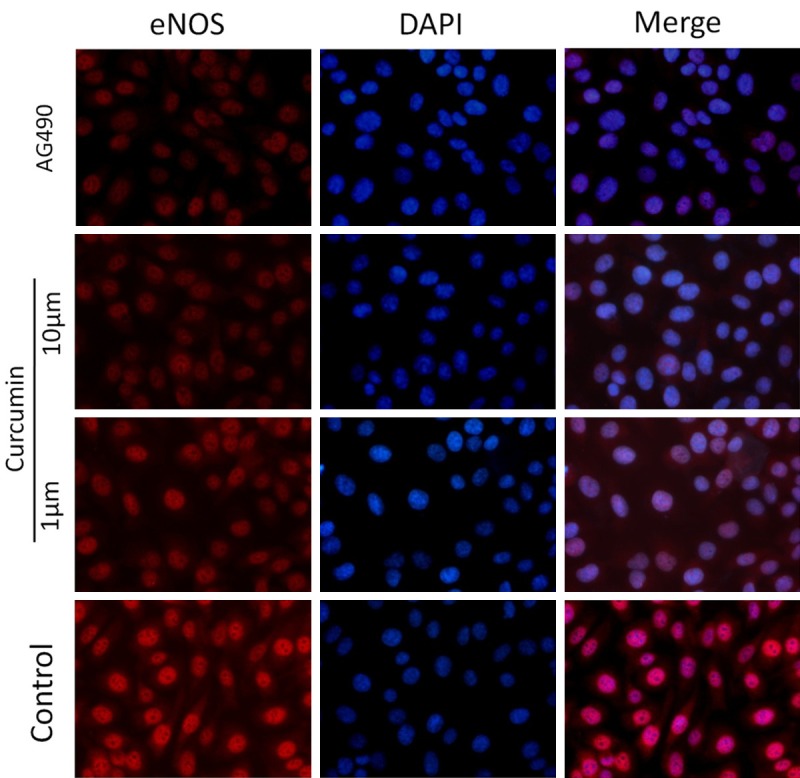
Effect of curcumin on eNOS expression in HEp-2 cells. Immunofluorescence staining of HEp-2 cells pretreated with either 1 μM/10 μM curcumin or 1 μM AG490 demonstrated inhibition of eNOS expression. Immunofluorescence staining for eNOS-positive cells revealed significantly difference in the treatment group compared to untreated controls.
Effect of curcumin on MMP-2 and VEGF activation in HEp-2 cells
Down-regulation of MMP-2 and VEGF expression by AG490 via JAK-2/STAT-3 dependent pathways has been shown in prostate cancer cells in vitro [23]. However, it is not known whether curcumin modulates the expression of MMP-2 and VEGF by inhibition of the JAK2/STAT3 pathway in HEp-2 cells. Thus, we examined the effect of curcumin on pSTAT3-induced activation of MMP-2 and VEGF in HEp-2 cells. To confirm whether curcumin inhibits the activation of MMP-2 and VEGF in HEp-2 cells, these cells were pretreated with curcumin (1 μM/10 μM). As shown in Figure 5, MMP-2 and VEGF was significantly decreased by curcumin pretreatment of HEp-2 cells. These results indicate that curcumin attenuates both MMP-2 and VEGF mRNA and protein expression.
Figure 5.
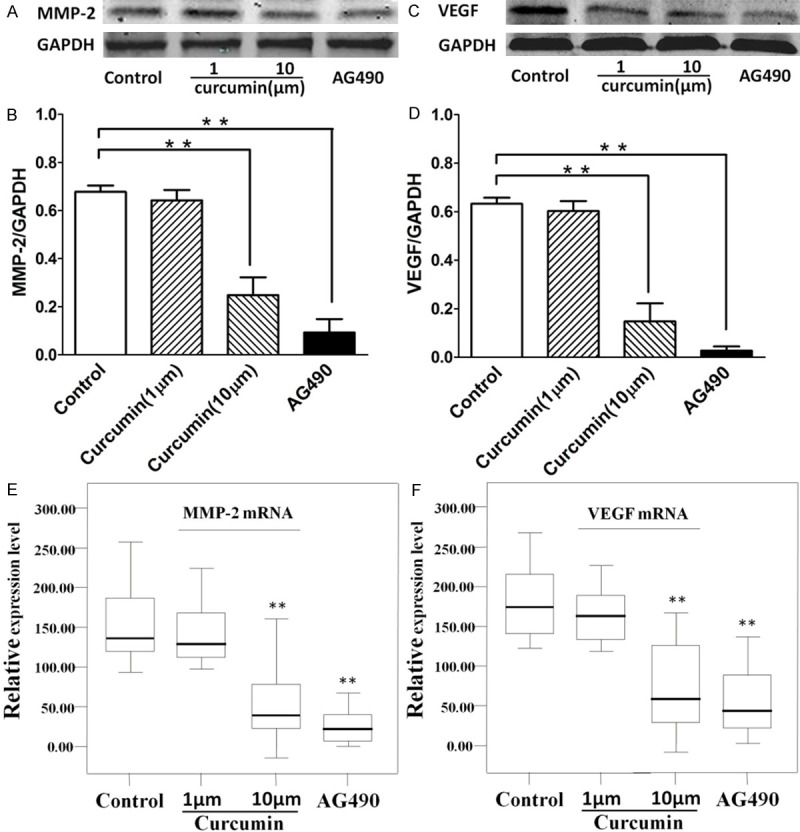
Effect of curcumin on MMP-2 expression and VEGF production in HEp-2 cells. We further investigated the effect of curcumin on MMP-2 expression and VEGF production in HEp-2 cells. HEp-2 cells were treated with curcumin for 5 h. Whole-cell lysates were subjected to western-blot analysis for determining levels of total MMP-2 and VEGF proteins. A and B. Inhibition of the JAK-2/STAT-3 pathways resulted in down-regulation and suppression of MMP-2 protein. Pretreatment of HEp-2 cells with either 1 μM curcumin, 10 μM curcumin or 1 μM AG490 significantly inhibited MMP-2 protein expression. C and D. Curcumin inhibits the activation of vasculogenic endothelial growth factor (VEGF) protein expression in HEp-2 cells. Pretreatment of HEp-2 cells with 10 μM curcumin resulted in inhibition of MMP-2 and VEGF production. E and F. indicated the effect of curcumin on MMP-2 and VEGF mRNA expression in HEp-2 cells. Semi-quantitative reverse transcription-PCR of MMP-2 and VEGF in HEp-2 cells demonstrates that MMP-2 and VEGF mRNA was down-regulated expression in HEp-2 cells. GAPDH was used as an internal loading control. The data are expressed as mean ± SD of three independent experiments, and significant differences from the control are indicated by **p < 0.01.
Discussion
VM (vasculogenic mimicry), a new pattern of blood supply to the tumor, has attracted the attention of many researchers, but many phenomena unique to VM channel formation remain to be elucidated. Tumor cell vasculogenic mimicry (VM) refers to the plasticity of aggressive cancer cells forming de novo vascular networks, which thereby contribute to perfusion of rapidly growing tumors, transporting fluid from leaky vessels, and/or connecting with the constitutional endothelial-lined vasculature [8]. VM has a totally different structure from endothelium-dependent vessels. Hence, traditional anti-vascular therapies aiming at endothelial cells have no remarkable effects on malignant tumor with VM. Anginex, TNP-470, and endostatin were effective angiogenesis inhibitors against endothelial cells but not against aggressive melanoma tumor cells with VM [19]. Similar results have been reported by Rybak et al. [24] in the B16F10 murine melanoma model with TNP470. When endothelium-dependent vessels have been inhibited by endostatin, VM may be do the job of endothelium-dependent vessels and maintain the tumor blood supply.
Aberrant activation of JAK/STAT3 signaling has been documented in a wide variety of human tumors, including hematopoietic malignancies and solid tumors such as head and neck, breast, and prostate cancers [25-27]. Constitutive STAT3 activation contributes to proliferation and oncogenesis by modulating the expression of a variety of genes required for tumor cell survival, proliferation, and angiogenesis, as well as invasion and metastasis and commonly suggests poor prognosis [23,28,29]. Thus, JAK/STAT3 signaling plays a central role in tumorigenesis and is considered an important therapeutic target for novel drug development. In this study, we demonstrate that curcumin exerts potent effects on the vasculogenic mimicry of laryngeal SCC, inhibiting multiple stages in the angiogenic process. We have demonstrated that (1) JAK-2 induction as well as pSTAT-3 production was blocked by curcumin; (2) AG490, a specific JAK-2 inhibitor, inhibited HEp-2 cells growth, proliferation, transmigration and tube formation.
Our findings suggest that curcumin exerts an inhibitory effect on laryngeal SCC line HEp-2 cells growth, proliferation, migration and tube formation, and suppresses angiogenesis by inhibiting JAK-2 expression and pSTAT-3 production. These data suggest that the inhibition of in vitro angiogenesis or reduced pSTAT-3 production will result from the inhibitory effect of curcumin on JAK-2 activation. Previously studies have demonstrated that JAK-2 induced pSTAT-3 expression, and pSTAT-3 production plays an important role in hepatocellular carcinoma cells angiogenesis [16]. In the present study, we demonstrate that curcumin inhibits the angiogenic effect of JAK-2 induced pSTAT-3 protein expression as well as eNOS production in HEp-2 cells. The inhibitory effect of curcumin on JAK-2 expression in HEp-2 cells indicates that curcumin is a potent inhibitor of JAK-2 expression and pSTAT-3 production in laryngeal SCCs. JAK-2 expression and pSTAT-3 production have been implicated as important mechanisms in angiogenesis during tumour growth, as STAT-3 is upregulated in the tumor microenvironment [30], and STAT-3 is found to be constitutively active in different carcinomas and inhibition of STAT-3 activation correlates with suppression of malignant cells both in vitro and in vivo [31,32]. In the present study, we demonstrate that the JAK-2 inhibitor AG490 impaired in vitro vasculogenic mimicry in HEp-2 cells, supporting the idea that JAK-2 derived pSTAT-3 play a central role in angiogenesis. Therefore, the inhibitory effect of curcumin could in part be the result of suppression of pSTAT-3 formation through inhibition of JAK-2 expression. In addition to the effect of curcumin on JAK-2 expression, curcumin also affects expression of several genes associated with cell growth and/or apoptosis (eg, eNOS). Curcumin inhibition of endothelial nitric oxide synthase (eNOS) expression in endothelial cells has been shown to contribute to impaired endothelial tube formation [22]. Taken together, our results suggest that the antiangiogenic activity of curcumin involves modulation of multiple pathways in laryngeal SCCs.
To date, the use of antiangiogenic agents in the treatment of human cancer has undergone limited exploration. The prototypical antiangiogenic agent thalidomide has been shown to benefit patients with refractory Crohn’s disease, but is fraught with problems, including its terrible legacy of causing severe birth defects during its early clinical use and unacceptable rates of neuropathy as an adverse reaction when employed on a long-term basis [33,34]. The potential for selective and non-selective JAK-2 antagonists as antiangiogenic agents for the treatment of laryngeal squamous cell carcinoma is also problematic, as these agents will typically worsen bowel injury in animal models of the disease. Therefore, the addition of curcumin, as an antiangiogenic agent which does not have the potential for clinical adverse side effects, may prove to be of significant benefit. At present, the majority of agents used for the treatment of laryngeal squamous cell carcinoma are felt to function through the inhibition of proliferation and migration, and the addition of an antiangiogenic compound may exert unique therapeutic benefit.
The STAT-3 is known to influence the expression of genes that promote migration and invasion [28]. Our results reveal that curcumin significantly reduced the expression of matrix metalloproteinases (MMP-2), the crucial players involved in the degradation of the extracellular matrix. The use of antiangiogenic agents in the treatment of gastrointestinal malignancies has emerged as the standard of care for metastatic lesions [35]. The anti-VEGF antibodies bevacizumab and cetuximab have proved successful in clinical trials, and optimal regimens for the use of these antiangiogenic agents in patients with metastatic colorectal adenocarcinoma are being defined [36]. The potential for “cocktails” of antiangiogenic agents which may target multiple mechanisms in the angiogenic process has also been shown. Therefore, the potential for the addition of curcumin in combination with other antiangiogenic strategies warrants evaluation.
In summary, our present study indicates that curcumin is a potent inhibitor of angiogenesis in HEp-2 cells in vitro. Curcumin appears to exert its antiangiogenic effect through inhibition of JAK-2 expression and pSTAT-3 production. Given the importance of angiogenesis and tumour neovascularization in cancer progression, our data also suggest that the anticancer effects of curcumin may also involve direct effects on vasculogenic mimicry. Future clinical studies evaluating the long-term benefit of curcumin as an antiangiogenic agent in the treatment of laryngeal squamous cell carcinoma are warranted.
Acknowledgements
This study was supported by grants from Shanghai Gongli Hospital Youth Project (No. 2013GLQN04) and Medical Project of Pudong New Area Health Bureau of Shanghai (No. PWZxq2014-09).
Disclosure of conflict of interest
None to declare.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ramroth H, Schoeps A, Rudolph E, Dyckhoff G, Plinkert P, Lippert B, Feist K, Delank KW, Scheuermann K, Baier G, Ott I, Chenouda S, Becher H, Dietz A. Factors predicting survival after diagnosis of laryngeal cancer. Oral Oncol. 2011;47:1154–1158. doi: 10.1016/j.oraloncology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 4.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci U S A. 2001;98:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sood AK, Fletcher MS, Coffin JE, Yang M, Seftor EA, Gruman LM, Gershenson DM, Hendrix MJ. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190:899–909. doi: 10.1016/j.ajog.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181:1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Sun B, Zhao X, Ma Y, Ji R, Gu Q, Dong X, Li J, Liu F, Jia X, Leng X, Zhang C, Sun R, Chi J. Twist1 expression induced by sunitinib accelerates tumor cell vasculogenic mimicry by increasing the population of CD133+ cells in triple-negative breast cancer. Mol Cancer. 2014;13:207. doi: 10.1186/1476-4598-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao L, Sun B, Zhao X, Gu Q, Dong X, Zheng Y, Sun J, Cheng R, Qi H, An J. Overexpression of Wnt5a promotes angiogenesis in NSCLC. Biomed Res Int. 2014;2014:832562. doi: 10.1155/2014/832562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao X, Dong X, Chi J. Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma. J Surg Oncol. 2013;108:414–419. doi: 10.1002/jso.23402. [DOI] [PubMed] [Google Scholar]
- 12.Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J, Sun T, Wang J, Sun R, Liu Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133:575–583. doi: 10.1016/j.ygyno.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Ruffini F, Graziani G, Levati L, Tentori L, D’Atri S, Lacal PM. Cilengitide downmodulates invasiveness and vasculogenic mimicry of neuropilin-1 expressing melanoma cells through the inhibition of alphavbeta5 integrin. Int J Cancer. 2015;136:E545–58. doi: 10.1002/ijc.29252. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2010;29:60. doi: 10.1186/1756-9966-29-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CH, Wu YT, Hsieh HC, Yu Y, Yu AL, Chang WW. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie. 2014;104:117–126. doi: 10.1016/j.biochi.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Chiablaem K, Lirdprapamongkol K, Keeratichamroen S, Surarit R, Svasti J. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition. Anticancer Res. 2014;34:1857–1864. [PubMed] [Google Scholar]
- 17.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 18.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 19.Huang MT, Ma W, Lu YP, Chang RL, Fisher C, Manchand PS, Newmark HL, Conney AH. Effects of curcumin, demethoxycurcumin, bisdemethoxycurcumin and tetrahydrocurcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion. Carcinogenesis. 1995;16:2493–2497. doi: 10.1093/carcin/16.10.2493. [DOI] [PubMed] [Google Scholar]
- 20.Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 21.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 22.Rossig L, Li H, Fisslthaler B, Urbich C, Fleming I, Forstermann U, Zeiher AM, Dimmeler S. Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ Res. 2002;91:837–844. doi: 10.1161/01.res.0000037983.07158.b1. [DOI] [PubMed] [Google Scholar]
- 23.Gurbuz V, Konac E, Varol N, Yilmaz A, Gurocak S, Menevse S, Sozen S. Effects of AG490 and S3I-201 on regulation of the JAK/STAT3 signaling pathway in relation to angiogenesis in TRAIL-resistant prostate cancer cells. Oncol Lett. 2014;7:755–763. doi: 10.3892/ol.2014.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybak SM, Sanovich E, Hollingshead MG, Borgel SD, Newton DL, Melillo G, Kong D, Kaur G, Sausville EA. “Vasocrine” formation of tumor cell-lined vascular spaces: implications for rational design of antiangiogenic therapies. Cancer Res. 2003;63:2812–2819. [PubMed] [Google Scholar]
- 25.Samsonov A, Zenser N, Zhang F, Zhang H, Fetter J, Malkov D. Tagging of genomic STAT3 and STAT1 with fluorescent proteins and insertion of a luciferase reporter in the cyclin D1 gene provides a modified A549 cell line to screen for selective STAT3 inhibitors. PLoS One. 2013;8:e68391. doi: 10.1371/journal.pone.0068391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa-Pereira AP, Bonito NA, Seckl MJ. Dysregulation of janus kinases and signal transducers and activators of transcription in cancer. Am J Cancer Res. 2011;1:806–816. [PMC free article] [PubMed] [Google Scholar]
- 27.Park JW, Zhao L, Cheng SY. Inhibition of estrogen-dependent tumorigenesis by the thyroid hormone receptor beta in xenograft models. Am J Cancer Res. 2013;3:302–311. [PMC free article] [PubMed] [Google Scholar]
- 28.Subramaniam A, Shanmugam MK, Perumal E, Li F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, Hui KM, Sethi G. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta. 2013;1835:46–60. doi: 10.1016/j.bbcan.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Denley SM, Jamieson NB, McCall P, Oien KA, Morton JP, Carter CR, Edwards J, McKay CJ. Activation of the IL-6R/Jak/stat pathway is associated with a poor outcome in resected pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013;17:887–898. doi: 10.1007/s11605-013-2168-7. [DOI] [PubMed] [Google Scholar]
- 30.Marino F, Orecchia V, Regis G, Musteanu M, Tassone B, Jon C, Forni M, Calautti E, Chiarle R, Eferl R, Poli V. STAT3beta controls inflammatory responses and early tumor onset in skin and colon experimental cancer models. Am J Cancer Res. 2014;4:484–494. [PMC free article] [PubMed] [Google Scholar]
- 31.Darnell JE Jr. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 32.Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, Karras JG, Levy DE, Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenpreis ED, Kane SV, Cohen LB, Cohen RD, Hanauer SB. Thalidomide therapy for patients with refractory Crohn’s disease: an open-label trial. Gastroenterology. 1999;117:1271–1277. doi: 10.1016/s0016-5085(99)70276-3. [DOI] [PubMed] [Google Scholar]
- 34.Vasiliauskas EA, Kam LY, Abreu-Martin MT, Hassard PV, Papadakis KA, Yang H, Zeldis JB, Targan SR. An open-label pilot study of low-dose thalidomide in chronically active, steroid-dependent Crohn’s disease. Gastroenterology. 1999;117:1278–1287. doi: 10.1016/s0016-5085(99)70277-5. [DOI] [PubMed] [Google Scholar]
- 35.Heidemann J, Binion DG, Domschke W, Kucharzik T. Antiangiogenic therapy in human gastrointestinal malignancies. Gut. 2006;55:1497–1511. doi: 10.1136/gut.2005.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Gramont A, Tournigand C, Andre T, Larsen AK, Louvet C. Adjuvant therapy for stage II and III colorectal cancer. Semin Oncol. 2007;34:S37–40. doi: 10.1053/j.seminoncol.2007.01.004. [DOI] [PubMed] [Google Scholar]


