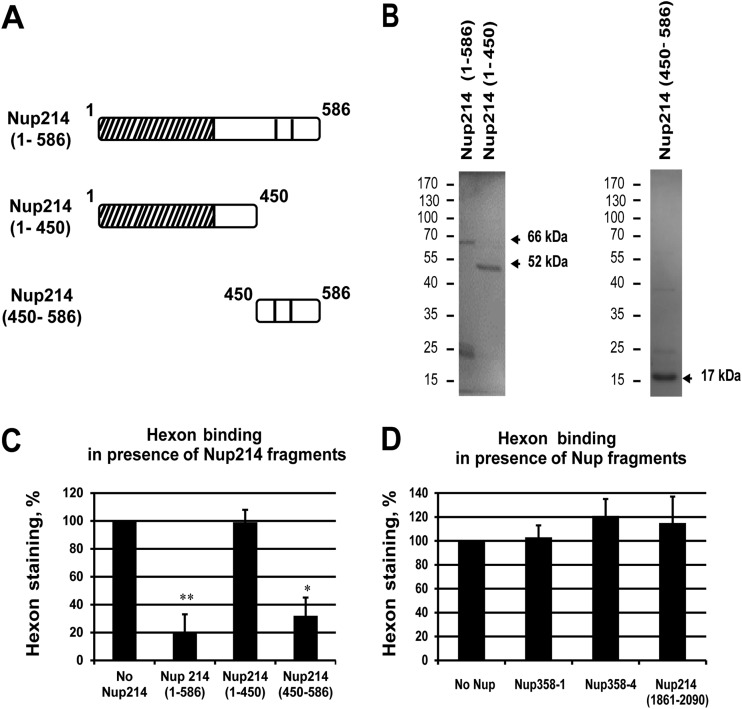
FIG 5.
Competition of hexon binding at the NE. Permeabilized HeLa cells (A, B, C, and D) or NRK cells (E) were incubated with purified hexon in transport buffer for 30 min in the presence or absence of soluble Nup fragments used as competitors and analyzed by IF staining using anti-hexon antibody. (A) Schematic representation of soluble Nup214 proteins used for hexon binding competition in HeLa cells as shown on Fig. 2A. (B) Expression of purified recombinant N-terminal proteins analyzed by SDS-PAGE and Coomassie staining. The expected size of each protein is indicated by the arrows. The migration and sizes of standard markers are shown on the left. (C) Quantitative analysis of hexon binding at the NE in HeLa cells in the presence of 2 μM concentrations of the soluble Nup214 proteins. The histogram shows the mean fluorescence intensity of hexon staining, indicated as a percentage, in the presence of Nup214(1–586), Nup214(1–450), or Nup214(450–586). The mean fluorescence intensity of hexon staining around the nucleus was measured (n = 36 to 57 cells for each condition of each experiment) and compared to the no-Nup214 condition, fixed at 100 (*, P < 0.05). (D) Representative analysis of hexon binding at the NE in HeLa cells in the presence of 2.5 μM concentrations of the soluble Nup proteins. The histogram shows the mean fluorescence intensity of hexon staining, indicated as a percentage, in the presence of Nup358-1 (aa 996 to 1963), Nup358-4 (aa 2500 to 3224), or Nup214 (aa 1861 to 2090). The mean fluorescence intensity of hexon staining around the nucleus was measured (n = 52 to 70 cells for each condition) and compared to the no-Nup condition, fixed at 100 (*, P < 0.05).