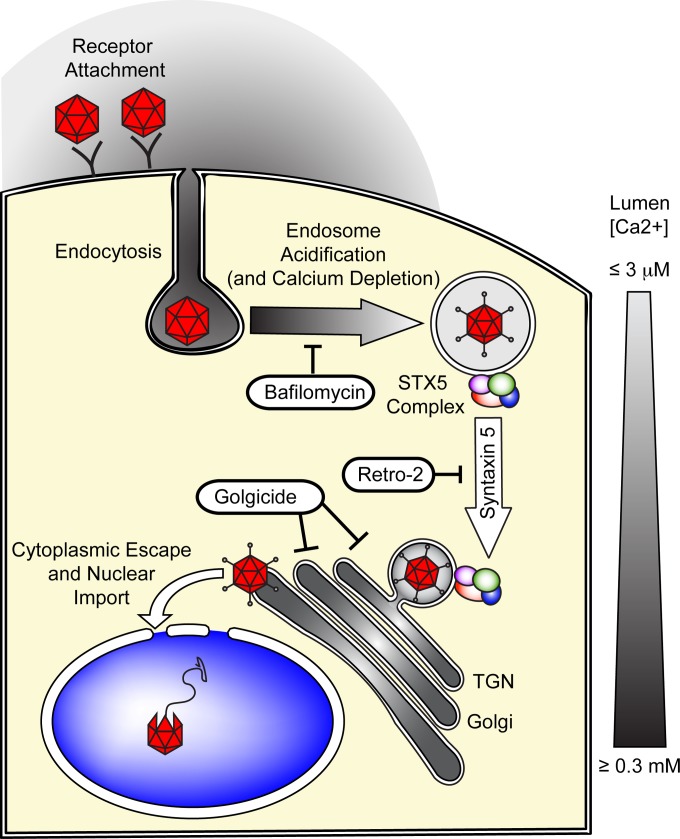
FIG 10.
Hypothetical model of syntaxin 5-dependent transport of AAV to the TGN/Golgi apparatus. Following receptor attachment and endocytosis, early endocytic vesicles are rapidly acidified, which can be inhibited by the proton ATPase inhibitor bafilomycin A1. This endosomal acidification is dependent on the depletion of endosomal calcium. The acidic environment of the endosomes and, presumably, the proteolytic cleavage of AAV capsid proteins trigger the extrusion of the unique, PLA2-containing region of the largest capsid protein, VP1. However, as a result of the minute calcium concentrations in the endosomes, the PLA2 is enzymatically inactive. AAV is then transported to the TGN/Golgi apparatus, and this transport can be inhibited with the GBF1 inhibitor golgicide. This endosome-to-TGN/Golgi apparatus transport step is also dependent on the tSNARE STX5, whose function can be inhibited by Retro-2 and its derivatives or by siRNA-mediated knockdown of STX5. The calcium concentration in the TGN/Golgi apparatus, which is near the optimal level for VP1-PLA2 activity, then allows escape into the cytoplasm, followed by nuclear import and uncoating of the AAV capsid.