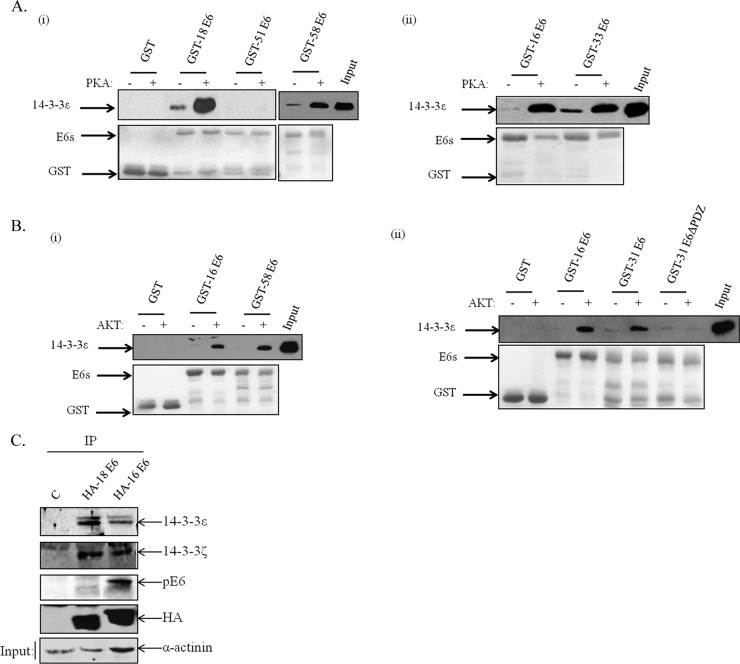
FIG 6.
Diverse HPV E6 oncoproteins interact with 14-3-3 in a phosphorylation-dependent manner. Different HPV E6-GST fusion proteins were purified and phosphorylated with either PKA (A) or AKT (B) in the presence of cold ATP. They were then incubated with purified 14-3-3ε, and after extensive washing, the bound His-tagged 14-3-3ε was detected by Western blotting with anti-6× His antibody. The upper portions show the results of the Western blots, while the lower portions show Ponceau staining of the nitrocellulose membranes confirming equal levels of protein loading. Arrows show the locations of the relevant proteins. (C) HEK293 cells were transfected with HA-tagged HPV-18 and HPV-16 E6 expression plasmids or empty plasmid. After 24 h, the cells were extracted and immunoprecipitated with anti-HA-conjugated agarose beads, and coprecipitating 14-3-3 isoforms were detected by Western blotting. Also shown is the anti-HA blot for total E6 and anti-phospho-E6.