Figure 1.
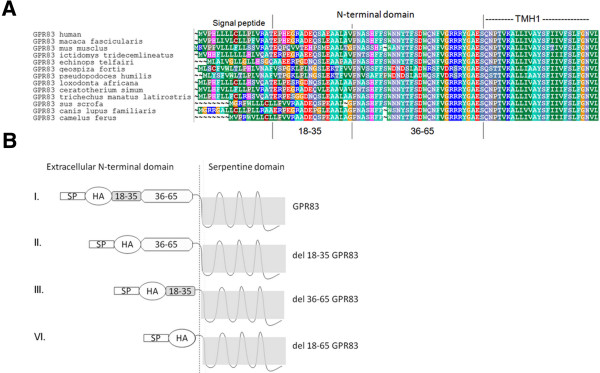
Sequence comparison of GPR83 othologs and designed GPR83 variants A) Alignment of N-terminal amino acids of GPR83 orthologs for comparison and identification of sequence conservation. Regions of conservation can be recognized by the overlapping colors. High conservation is also indicative for a specific fold and/or function. It is evident that especially the second half of the N-terminal tail (between positions 36–65) is highly conserved among the compared variants. Different colors of amino acids indicate their biophysical properties: green – hydrophobic, blue – positively charged, red – negatively charged. The alignment was visualized using BioEdit. B) Schematic representation of GPR83 deletion mutants: The experimentally deleted parts are highlighted: a. deletion 18–35, b. deletion 36–65, c. deletion 18–65. The position of the signal peptide is indicated as SP and the hemagglutinin tag with HA.
