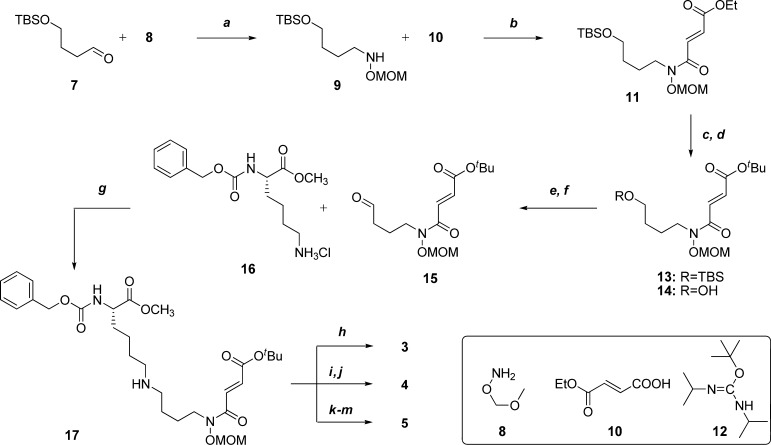
Scheme 1. Synthesis of mono-, di-, and tri-methylated lysine analogs 3–5.
Reagents and conditions: (a) 7 (1.0 equiv), MeOH, 25 °C, 20 min; then NaBH3CN (1.0 equiv/h for 6 h), acetic acid (1 eqiv/h for 6 h), MeOH, 25 °C, 6 h, 51%; (b) 10 (1.5 equiv), oxalyl chloride (2.0 equiv), DMF (0.050 equiv), CH2Cl2, 0–25 °C, 1 h; then 9, Et3N (3.0 equiv), DMAP (0.010 equiv), CH2Cl2, 0 °C, 10 min, 89%; (c) LiOH (1.0 equiv), THF/H2O, 25 °C, 2.5 h, 99%; (d) 12 (3.0 equiv), 1,4-dioxane, 50 °C, 16 h, 68%; (e) TBAF (1.5 equiv), THF, 0–25 °C, 16 h, 96%; (f) oxalyl chloride (2.0 equiv), DMSO (4.0 equiv), CH2Cl2, −78 °C, 30 min.; then 14, CH2Cl2, −78 °C, 15 min.; then Et3N (6.0 equiv), CH2Cl2, −78–25 °C, 15 min, 98%; (g) DIEA (2.0 equiv), 16 (2.0 equiv), MeOH, 25 °C, 2 h; then NaBH3CN (2.0 equiv), acetic acid (1.0 equiv), MeOH, 25 °C, 16 h, 58%; (h) TFA/TIPS/H2O 95:2.5:2.5, 25 °C, 4.5 h, 75%; (i, k) CH2O (2.0 equiv), EtOH, 25 °C, 2 h; then NaBH3CN (2.0 equiv), acetic acid (1.0 equiv), EtOH, 25 °C, 16 h, 38%; (j) TFA/TIPS/H2O 95:2.5:2.5, 25 °C, 4.5 h, 49%; (l) MeI/MeOH 1:1, 25 °C, 2 d, 98%; (m) TFA/TIPS/H2O 95:2.5:2.5, 25 °C, 4.5 h, 99%.