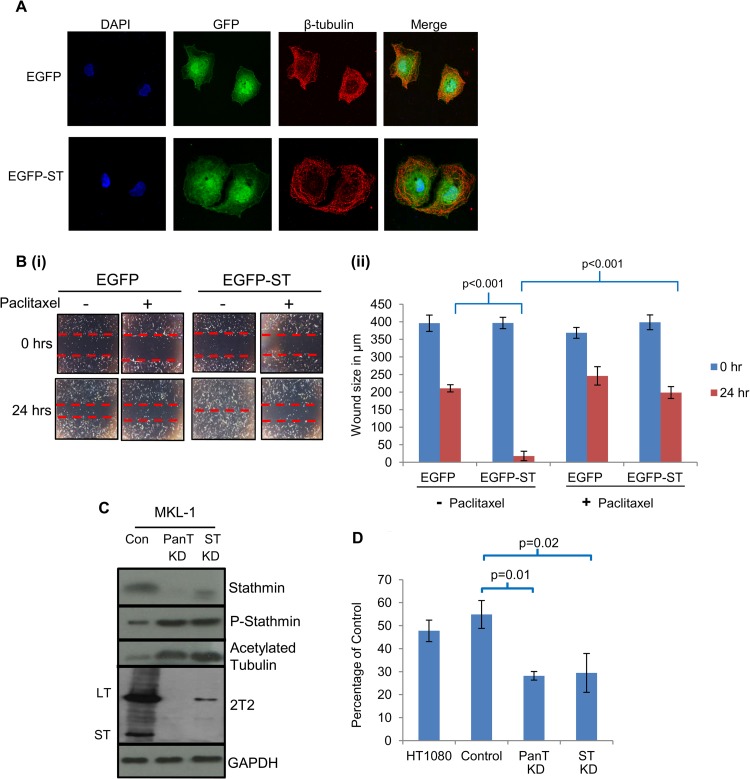
FIG 5.
Microtubule destabilization is required for MCPyV ST-mediated cell motility. (A) MCC13 cells expressing EGFP or EGFP-ST were incubated in the presence of paclitaxel. After 24 h, cells were fixed and permeabilized, and GFP fluorescence was analyzed by direct visualization, whereas endogenous β-tubulin was identified by indirect immunofluorescence using a β-tubulin-specific antibody. (B) (i) MCC13 cells were transfected with either EGFP or EGFP-ST expression vectors and also incubated in the absence or presence of paclitaxel. After 24 h, a scratch was created by scraping the monolayer, migration of cells toward the scratch was observed over a 24-h period, and images were taken at 0 and 24 h under a Zeiss light microscope at ×4 magnification. (ii) The size of the wound was measured at 0 and 24 h. Scratch assays were performed in triplicate. (C) (i) MKL-1 cells were transduced with lentivirus-based vectors expressing shRNAs targeting ST alone, both LT and ST (PanT), and a scrambled negative control (Con). Cellular lysates were then analyzed by immunoblotting using stathmin-, phosphorylated stathmin-, acetylated tubulin-, and MCPyV T antigen (2T2)-specific antibodies. GAPDH was used as a measure of equal loading. (D) Precoated Matrigel-based Transwell migration tissue culture plates were seeded with MKL-1 cells transduced with scrambled (control)-, ST-, or ST-LT (PanT)-targeting lentivirus-based vectors. HT1080 cells, an invasive human fibrosarcoma cell line, were also used as a positive control. Cells were incubated in the Transwell plates for 22 h and then labeled with calcein AM fluorescent dye for 90 min, and fluorescence was measured. The experiment was performed in triplicate, and the graph indicates the fluorescence (percentage) of cells moving through the migration plates relative to the uncoated plate, set at 100%.