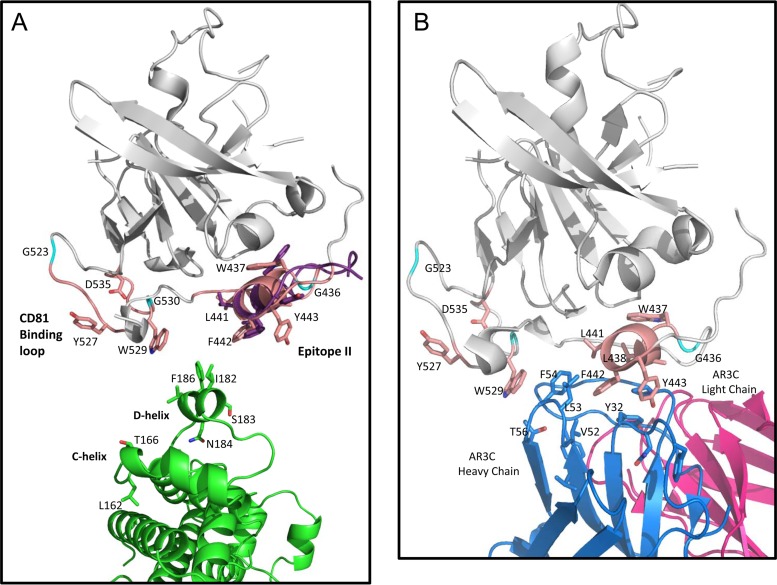
FIG 9.
(A) Cartoon representation of the docked conformer of epitope II peptide superimposed with the E2 core structure (PDB ID 4MWF). The E2 core is shown in gray, with regions involved in CD81 binding highlighted pale pink and specific side chains associated with CD81 binding displayed as follows: carbons in pale pink, oxygens in red, and nitrogens in blue, with the exception of glycines, which are shown in cyan. The docked epitope II peptide conformer superimposed on the same sequence in E2 is shown with side chains displayed in stick representation, with carbons shown in purple, oxygens in red, and nitrogens in blue. The CD81-LEL dimer is shown with the same color scheme as that denoted in the legends to Fig. 7 and 8, with the side chains of the D-helix shown in stick representation and positioned as a reference to how this region may interact with the E2 helix motif, W437LAGLFY443. (B) Cartoon representation of the published E2 core structure in complex with antibody AR3C (PDB ID 4MWF), with residues of the HC CDR loop regions of AR3C that show similarity to the D-helix of CD81 displayed. The color scheme for the E2 core is the same as that described for panel A. The light chain of AR3C is shown in magenta, the heavy chain is shown in blue, and residues of the HC CDR2 loop (including residues V52, L53, and F54) and the CDR1 loop (including residue Y32) are shown in stick representation, with carbons in blue and oxygens in red.