Abstract
The oncogenic γ-herpesviruses EBV and KSHV are ubiquitous human pathogens that establish lifelong latent infections maintained by intermittent viral reactivation and reinfection. Effector CD4 T cells are critical for control of viral latency and in immune therapies for virus-associated tumors. Here we exploited γHV68 infection of mice to enhance our understanding of the CD4 T cell response during γ-herpesvirus infection. Using a consensus prediction approach, we identified 16 new CD4 epitope-specific responses that arise during lytic infection. An additional epitope encoded by the M2 protein induced uniquely latency-associated CD4 T cells, which were not detected at the peak of lytic infection but only during latency, and were not induced after infection with a latency-deficient virus. M2-specific CD4 T cells were selectively cytotoxic, produced multiple antiviral cytokines, and sustained IL-2 production. Identification of latency-associated cytolytic CD4 T cells will aid in dissecting mechanisms of CD4 immune control of γ-herpesvirus latency and the development of therapeutic approaches to control viral reactivation and pathology.
Introduction
A majority of people worldwide are infected with the oncogenic human γ-herpesviruses (γHVs), EBV and Kaposi’s sarcoma-associated herpesvirus (KSHV), making these viruses a considerable public health concern. After an initial acute infection, γHVs are maintained lifelong in a latent state within cells of the immune system. Under conditions of immune suppression, such as following transplantation or HIV infection, γHVs can reactivate from latency, leading to recrudescent disease and the development of cancers. Cytotoxic CD4 T cells play an important role in immune control of the γHVs in part because viral immune evasion mechanisms impair CD8 T cell recognition by down-regulating MHC-I molecules (1), and the major viral latency reservoir is within MHC-II+ B cells (2). Decline of CD4 T cell immunity to EBV correlates with the development of EBV-associated cancers, including Hodgkin’s disease, nasopharyngeal carcinoma and Burkitt’s lymphoma, and CD4 T cells have also been used therapeutically for treatment of EBV-associated malignancies (3–5). Here, we have infected mice with murine γHV68 to study antiviral CD4 T cell responses to acute and latent γHV infections, as the human γHVs are highly species-specific, making detailed in vivo kinetic studies of the host immune response difficult. We identify 16 new epitopes during acute infection that promote cytokine-producing CD4 T cell responses. These responses exhibit differential kinetics during the early stages of latency establishment with some responses expressed only transiently and others maintained throughout stable latency. Infection with a latency-deficient virus shows that the long-term maintenance of epitope-specific CD4 T cell responses, but not their initial generation, is dependent on latency establishment. Expression of an additional epitope from the latency-associated M2 protein is unique, in that it does not stimulate cells during acute infection, but only after the establishment of latency. M2-specific CD4 T cells sustain IL-2 production in addition to IFNγ and TNFα, and exhibit potent killing of MHC-II-expressing cells in vivo. This study substantially broadens our understanding of the CD4 T cell response to γHV68 infection and identifies a valuable latency-associated target for rational vaccine design.
Materials and Methods
Mice and virus infections
C57BL/6 and B6.SJL-PtprcaPepcb/Boy mice were bred at Trudeau Institute and kept under specific-pathogen free conditions. Mice were anesthetized with 2,2,2-tribromoethanol and infected intranasally with 400–800 PFU γHV68 (strain WUMS) or AC-RTA (6) (a gift from R. Sun and T.-T. Wu, UCLA). All experiments were approved by the Institutional Animal Care and Use Committee of the Trudeau Institute.
Peptide prediction
Protein sequences were analyzed using previously described algorithms (7) that predict the affinity of 15-mer peptides for I-Ab class II molecules. Briefly, all 15-mer peptides that are encoded in open reading frames (ORFs) of the γHV68 genome (GenBank NC_001826) were predicted for binding to H-2 I-Ab. Two independent algorithms (ARB (8) and SMM-Align (9)) based on positional scoring matrices were used to assign predicted IC50 binding affinities to all peptides. For each method, peptides were ranked by their predicted binding affinity, and the median of the two ranks was used to select the top 680 out of 34,008 peptides (top 2%). Peptides overlapping by 9 or more residues were excluded. Peptides were synthesized by Mimotopes (Clayton, Victoria, Australia).
T cell function assays
Intracellular cytokine staining, functional avidity analysis, and ELISpot assay for IFNγ expression were performed as described previously (10). For the combined intracellular cytokine staining and BrdU incorporation assay, mice were treated in vivo with a single i.p. injection of 250 μg BrdU and 0.8 mg/ml BrdU in the drinking water for 4 d (11). The in vivo cytotoxicity assay was performed essentially as described previously (10). Experimental peptide-pulsed splenocytes were labeled with differential concentrations of CFSE and control peptide (influenza NP311-325)-pulsed cells were left unlabeled. Cells were mixed in a 1:1:1 ratio (M2124-138:ORF75b1020-1034:NP311-325) and a total of 6 × 107 cells were intravenously injected into mice that had been previously infected with WT γHV68 or AC-RTA (or into naive mice to calculate specific lysis). Approximately 16 h post-injection CD45.1+MHC-II+CFSE+ cells were enumerated by flow cytometry. Samples were collected on a BD FACSCanto II cytometer and analyzed using FlowJo software (TreeStar). Specific lysis was calculated using the formula: [1 − (ratio uninfected/ratio infected)] × 100.
M2124-138-specific tetramers
MHC class II-restricted tetramers expressing M2124-138 peptide (NSEPVYIQPISTRSL) were generated by the NIH tetramer core facility. To identify M2124-specific CD4 T cells ex vivo, cells were incubated with 6μg/ml tetramer at 37°C for 1.5h. MHC class I-restricted tetramers expressing ORF61524-531 peptide (TSINFVKI) were generated and used as previously described (10).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5 software (San Diego, CA). Differences were considered significant at P values less than 0.05.
Results
Identification of novel γHV68-specific CD4 T cell epitopes
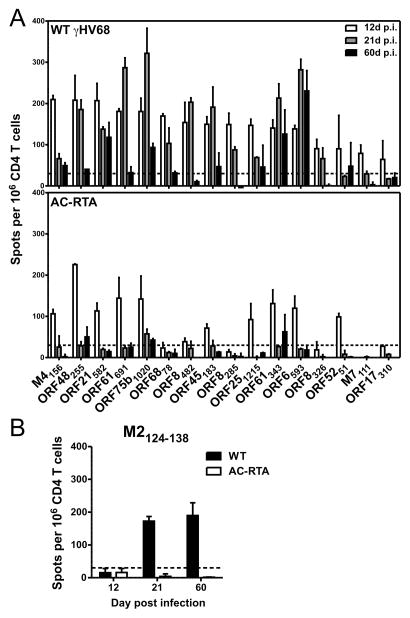
CD4 T cells are important in controlling persistent viral infections, not just as “helpers” for CD8 T cells, but also as cytokine-secreting and cytotoxic effector cells. Our understanding of the breadth of the virus-specific CD4 T cell response to γHV68 in C57BL/6 mice has been largely limited to two epitopes, from glycoprotein 150 (gp15067-83) and ORF11 (ORF11168-180) (12, 13). To identify additional CD4 T cell epitopes, we used an MHC-II-binding prediction algorithm to identify and synthesize 680 15-mer peptides from γHV68 genome coding sequences (7, 14, 15). As we observed previously when we used a similar algorithm to predict CD8 epitopes (10), there was a strong correlation between protein size and the number of predicted epitopes (r2=0.6909, P≤0.0001; Pearson correlation). Each peptide was screened for its ability to stimulate IFNγ by splenic CD4 T cells from mice 12 d after wild-type (WT) γHV68 infection. We initially used peptide pools (up to 8 peptides per pool) to narrow down the number of candidate peptides, then deconvoluted the positive pools and tested each individual peptide. We identified 16 peptides that reproducibly elicited IFNγ responses at 12 d post infection (p.i.) (Fig. 1A, top). The 16 peptides were derived from 13 proteins, mainly encoded by early-late and late genes (Table I) (16), consistent with recent reports on the specificity of CD4 T cells to EBV and KSHV (17–19). As has been observed in KSHV infection (18), several proteins that elicit γHV68-specific CD8 T cell responses, including ORF6, ORF8, ORF17, ORF48, and ORF61, also stimulated CD4 T cell responses; the ORF17310-324 (ITNHAAFSTPGAGLS) sequence overlapped with a known CD8 epitope, ORF17308-316 (SAITNHAAF) presented by H-2Db (10, 20). Eight of the 16 peptides elicited sustained (≥70% of the day 12 production) or enhanced IFNγ responses at 21 d p.i., suggesting there is differential regulation of epitope-specific CD4 T cell responses during the latency amplification phase, similar to antiviral CD8 T cell responses (21). Most of the responses were markedly reduced at 60 d p.i., consistent with memory CD4 T cells having undergone substantial contraction.
Figure 1. Robust IFNγ production by novel epitope-specific CD4 T cell responses.
(A) IFNγ production by CD4 T cells at 12, 21, and 60 d p.i. with WT γHV68 (top) or AC-RTA (bottom). (B) IFNγ production by CD4 T cells after stimulation with the M2124-138 peptide after infection with WT γHV68 or AC-RTA. The dotted lines are at 30 spots above background. Samples were run in duplicate and are from at least 2 experiments per time point.
Table I.
Novel CD4 T cell epitopes.
| ORF | Amino Acids | Sequence | Function | Gene Expressiona | Stimulated by AC-RTA (12 d p.i.) |
|---|---|---|---|---|---|
| M2 | 124–138 | NSEPVYIQPISTRSL | Immune regulation/latency | E-L | No |
| M4 | 156–170 | RSSQWEVAFSVVSKT | Immune regulation | IE | Yes |
| ORF6 | 593–607 | CNTNWLPPCPIIHNL | ssDNA binding protein | E-Lb | Yes |
| ORF8 | 285–299 | GQSRTFLETPSATYS | glycoprotein B | L | No |
| ORF8 | 326–340 | HEHSYHFVANEVTAT | glycoprotein B | L | No |
| ORF8 | 482–496 | TLMWYELSKVNPTSV | glycoprotein B | L | Yes |
| ORF17 | 310–324 | ITNHAAFSTPGAGLS | Serine protease/capsid morphogenesis | L | No |
| ORF21 | 582–596 | LLHKFCYELSKPHMV | Thymidine kinase | E-L | Yes |
| ORF25 | 1215–1229 | KLLYDHGQPDPAYEF | Major capsid protein | E-L | Yes |
| ORF45 | 183–197 | ESSRILKTPAPISGN | Tegument protein | L | Yes |
| ORF48 | 255–269 | DTSSTWTWPAARIAE | Tegument protein | L | Yes |
| M7 | 111–125 | PTEADPKAAPSAGHV | glycoprotein 150 | L | No |
| ORF52 | 51–65 | SIIVSSSRALGAVAM | Tegument protein | L | Yes |
| ORF61 | 343–357 | EQGAYEAVVPIKSVM | Ribonucleotide reductase large subunit | L | Yes |
| ORF61 | 691–705 | LFLNEDYASSASNIK | Ribonucleotide reductase large subunit | L | Yes |
| ORF68 | 78–92 | LCFYVLHAPVTWSAT | DNA packaging protein | E | No |
| ORF75b | 1020–1034 | MLQYAGFLPEIVHSS | Tegument protein | E | Yes |
We next analyzed IFNγ production after infection with AC-RTA, a recombinant γHV68 that induces robust peak lytic titers in the lungs (similar to or greater than WT virus) but cannot establish latency (6, 10). Only 11 of the 16 peptides elicited IFNγ responses above background 12 d after infection with AC-RTA (Fig. 1A, bottom and Table I). AC-RTA does not reliably traffic to the spleen, and so would result in negligible systemic viral loads after acute infection. Therefore, these data suggest the initial breadth of the CD4 T cell response may be governed at least in part by antigen presentation during latency amplification in the spleen. Accordingly, none of the CD4 T cell responses were sustained or increased at 21 d, or were maintained 60 d after AC-RTA infection (Fig. 1A, bottom). These results also suggest that antiviral CD4 T cell responses generated during acute infection are more reliant on prolonged antigen presentation than their antiviral CD8 T cell counterparts (10). We then tested the peptide pools at later times after infection to identify epitopes that might arise during latency. Notably, one peptide (M2124-138) that did not elicit IFNγ production above background 12 d p.i. induced robust IFNγ production at 21 and 60 d after infection with WT γHV68, but not AC-RTA (Fig. 1B). Extrapolating from the ELISpot data, the M2124-138-specific response in WT γHV68-infected mice accounted for 0.65% of the total CD4 T cell response at 12 d, 7.0% at 21 d, and 17.5% at 60 d p.i. Expression of the M2 gene is temporally-restricted and associated with latent infection (22, 23). The M2 protein is key in the establishment of, and reactivation from, viral latency, and drives the differentiation of infected B cells (24–26). M2 also encodes an MHC-I-restricted epitope that induces a latency-associated CD8 T cell response in Balb/c mice (22). Thus, we have identified a novel, latency-associated CD4 T cell epitope within M2 that stimulates robust IFNγ expression as late as 60 d p.i.
γHV68-specific CD4 T cells are polyfunctional cytokine producers
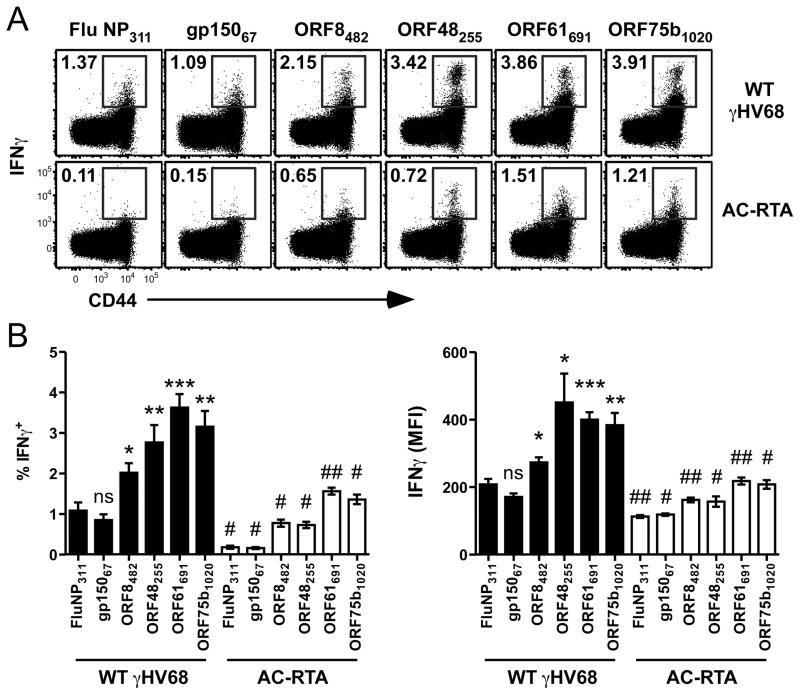
Early studies described an IFNγ-mediated role of CD4 T cells in maintaining γHV68 latency (27). Therefore, we set out to analyze the cytokine secretion profile of epitope-specific CD4 T cells throughout infection. We observed robust ex vivo IFNγ production by splenic CD4 T cells from γHV68-infected mice 12 d p.i. regardless of whether the cells were stimulated with positive control gp15067-83 peptide or negative control influenza NP311-325 peptide (Fig. 2A). These data confirm the previous observation that CD4 T cells isolated from γHV68-infected mice produce substantial IFNγ in a nonspecific manner when assessed by intracellular cytokine staining (28). When cells were stimulated with the newly-identified peptides ORF8482-496, ORF48255-269, ORF61691-705, or ORF75b1020-1034, however, we detected significantly greater IFNγ production than the nonspecific or gp15067-83 peptide-induced levels (Fig. 2A and B). These data strongly suggest that at least some antigen-driven stimulation of γHV68-specific CD4 T cells elicits IFNγ production above the nonspecific “background” level. Notably, stimulation of CD4 T cells with each peptide induced substantially less IFNγ after AC-RTA infection than WT infection (Fig. 2A and B). It is unclear why the gp15067-83 peptide does not stimulate more robust IFNγ expression in this assay, given it was originally identified by its ability to stimulate IFNγ production (12).
Figure 2. Epitope-specific IFNγ production.
(A) Representative dot plots showing IFNγ production and CD44 expression by splenic CD4 T cells measured by intracellular cytokine assay 12 d after WT γHV68 (top row) or AC-RTA (bottom row) infection (n=5, representative of 2 experiments). (B) The percent of CD4 T cells that are IFNγ+ (left) or the mean fluorescence intensity (MFI) of IFNγ expression by CD4 T cells (right) in the spleen 12 d after WT γHV68 or AC-RTA infection (n=5, representative of 4 experiments; ns, not significant; *P≤0.05; **P≤0.01; ***P≤0.001; Student’s t test, compared to control values; #P≤0.01; ##P≤0.001; Student’s t test, compared AC-RTA to WT values).
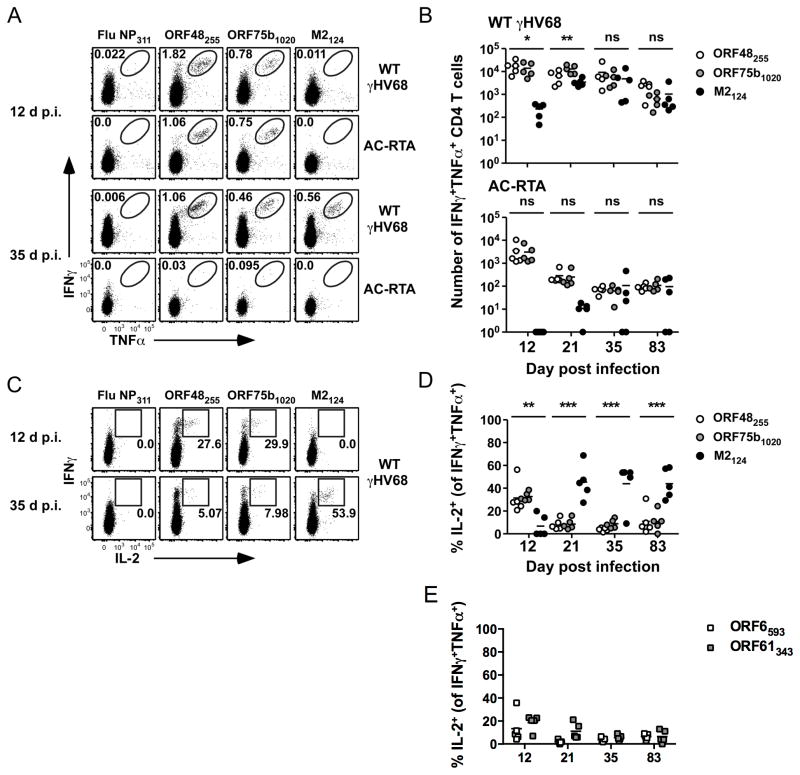
We also observed robust ex vivo co-production of IFNγ and TNFα by lung CD4 T cells from γHV68-infected mice 12 d p.i. (Fig. 3A and B). Stimulation of cells from AC-RTA-infected mice with the ORF48255-269 and ORF75b1020-1034 peptides elicited IFNγ and TNFα production 12 d but not 35 d p.i., consistent with the ELISpot results (Fig. 2). At 12 d p.i. we could not detect cytokine co-production stimulated by the M2124-138 peptide in either WT γHV68- or AC-RTA-infected mice, but did detect robust cytokine production by M2124-138-specific cells 35 d after WT γHV68 infection (Fig. 3A). The number of dual-cytokine producing M2124-138-specific cells peaked at 21 d p.i. and was maintained long-term (Fig. 3B). In the absence of viral latency (i.e. severely reduced systemic viral loads after acute infection is cleared) we could detect very low numbers of cells specific for M2124-138 in some mice (Fig. 3B). This may reflect differential sensitivities of intracellular cytokine staining and ELISpot analyses, and is likely due to the fact that M2 gene expression is not exclusively latent, but is expressed during acute infection, and that the AC-RTA virus does express M2 (6).
Figure 3. Epitope-specific CD4 T cells are polyfunctional.
(A) Representative dot plots showing IFNγ and TNFα production by lung CD4 T cells at 12 or 35 d after WT γHV68 or AC-RTA infection. Numbers in the plots indicate the percent of CD4 T cells in the gate. (B) The number of IFNγ+TNFα+ CD4 T cells in the lungs over time after WT γHV68 or AC-RTA infection (n=5, representative of 4 experiments; ns, not significant; *P≤0.05; **P≤0.01; one-way ANOVA). (C) Representative dot plots showing IFNγ and IL-2 production by CD4 T cells at 12 days or 35 days after WT γHV68 infection. Numbers in the plots indicate the percent of IFNγ+TNFα+ CD4 T cells expressing IL-2. (D, E) The percent of IFNγ+TNFα+ CD4 T cells that are IL-2+ in the lungs specific for the indicated antigens over time after WT γHV68 infection (n=5, representative of 4 experiments; **P≤0.01; ***P≤0.001; one-way ANOVA).
CD4 T cell polyfunctionality (i.e., expression of IFNγ, TNFα, and IL-2) is associated with improved protection after vaccination or secondary challenge (29, 30), so we next tested whether IFNγ+TNFα+ CD4 T cells were capable of producing IL-2 after peptide-specific stimulation (Fig. 3C and D). We observed considerable IL-2 production by ORF48255-269-specific and ORF75b1020-1034-specific IFNγ+TNFα+CD4 T cells in the lungs 12 d p.i. IL-2 expression declined rapidly, and by 21 d p.i. these cells produced very little IL-2 (Fig. 3D). IL-2 production also declined in two responses, ORF6593-607 and ORF61343-357 (Fig. 3E), that had sustained IFNγ production by ELISpot analysis (Fig. 1A). Conversely, M2124-138-specific CD4 T cells exhibited marked expression of IL-2 that persisted at least 83 d. Thus, early in infection, virus-specific CD4 T cells demonstrate polyfunctionality – the combined production of IFNγ, TNFα, and IL-2. These data are in contrast to previous observations showing that γHV68-specific CD4 T cells that secreted IFNγ did not make TNFα or IL-2 (28). After the latency amplification phase, only M2124-138-specific CD4 T cells exhibit sustained IL-2 production, suggesting that polyfunctional M2124-138-specific CD4 T cells may play an important role in protection from viral reactivation.
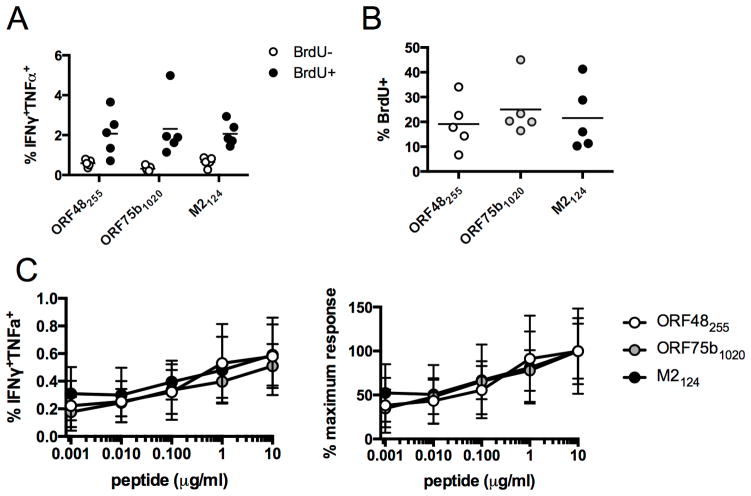
We next sought to determine whether there were appreciable differences in the cell cycling or proliferation of lytic or latent epitope-specific CD4 T cells, using a strategy of combining in vivo BrdU treatment with ex vivo stimulation and intracellular cytokine staining (11). Two months after infection, we observed considerable enrichment of epitope-specific CD4 T cells in the BrdU-positive (proliferating) population (Fig. 4A), but we did not observe differential BrdU incorporation in IFNγ+TNFα+CD4 T cells specific for ORF48255-269, ORF75b1020-1034, or M2124-138 (Fig. 4B). Notably, we also did not observe any differences in the functional avidity of lytic or latent antigen-specific CD4 T cells. Each population synthesized IFNγ at similarly low concentrations of peptide stimulation (Fig. 4C).
Figure 4. Proliferation of cytokine-expressing antiviral CD4 T cells.
(A, B) At 56 d p.i. mice were treated with BrdU in their drinking water for 4 d. (A) The percent of BrdU+ or BrdU− CD44hi CD4 T cells that are IFNγ+TNFα+. (B) The percent of IFNγ+TNFα+ CD4 T cells that are BrdU+. (C) IFNγ and TNFα co-expression following stimulation with varying concentrations of ORF48255, ORF75b1020, or M2124 epitopes. Data shown are raw values (left graph) or expressed as the percentage of the 10 μg/ml value (right graph) (n=5/epitope, representative of 2 experiments).
M2124-138-specific CD4 T cells are cytotoxic in vivo
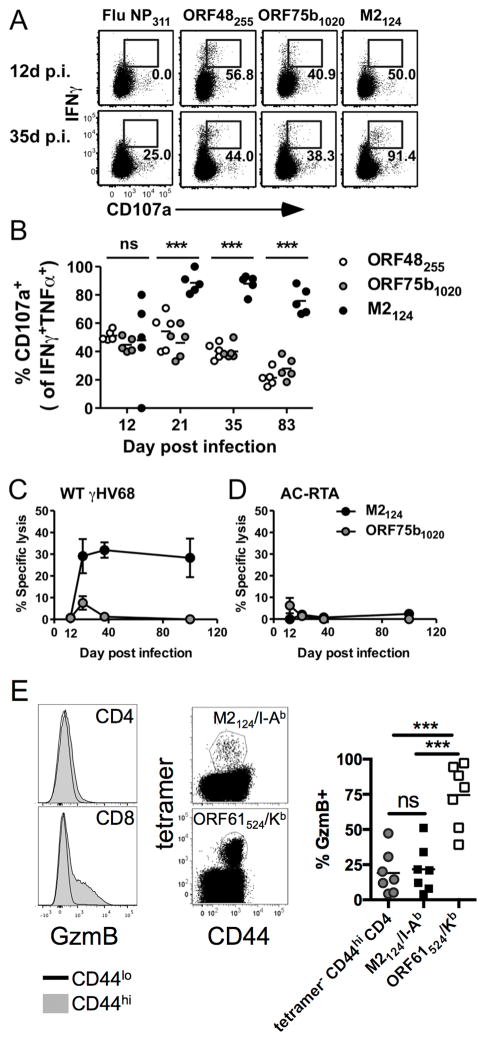
CD4 T cell cytotoxicity has been described after γHV68 infection, although no antigen-specificity for this effect was identified (28, 31). To determine if any of the CD4 T cells specific for the newly identified γHV68 epitopes possessed cytotoxic function, we first measured CD107a expression after ex vivo antigen stimulation. CD107a expression correlates with the ability of antigenic stimulation to induce the release of lytic granules (32). At 12 d p.i., about half of the antigen-specific CD4 T cells from the lungs expressed CD107a after stimulation, and the percentage of IFNγ+TNFα+ cells specific for ORF48255-269 and ORF75b1020-1034 that expressed CD107a declined over time (Fig. 5A and B). In contrast, once M2124-138-specific CD4 T cells had substantially accumulated in the lungs, nearly all of them expressed CD107a on the cell surface following stimulation for up to 83 d after infection (Fig. 5A and B). Next, we directly assessed the cytotoxic ability of antigen-specific CD4 T cells in vivo. At 12 d after WT γHV68 or AC-RTA infection, there was negligible killing of target cells pulsed with either the M2124-138 or ORF75b1020-1034 peptides. Beginning 21 d after WT γHV68 infection, and continuing at least 100 d, about 30% of M2124-138-pulsed targets were killed while ORF75b1020-1034-specific killing remained very low (Fig. 5C), even though the two epitope-specific responses had similar frequencies in the antiviral CD4 T cell pool after 21 d (Fig. 3B). We did not detect killing of M2124-138-pulsed targets in AC-RTA-infected mice (Fig. 5D). Thus, CD4 T cells specific for the latency-associated M2124-138 epitope that arise after the initial acute infection but concurrent with the amplification of viral latency are polyfunctional and cytotoxic. These findings argue against the previous general conclusion that IFNγ-producing and cytotoxic γHV68-specific CD4 T cells are functionally distinct populations (28, 31). We do not yet know the mechanism by which M2124-138-specific CD4 T cells kill their targets, and our data suggest it may not be entirely via lytic granule release. CD44hi CD4 T cells express much less granzyme B than CD44hi CD8 T cells (Fig. 5E, left). Intracellular granzyme B levels were significantly lower in M2124-138-specific CD4 T cells than cytotoxic ORF61524-531-specific CD8 T cells, and were not enriched in M2124-138-specific CD4 T cells relative to the remaining tetramer-negative CD44hi CD4 T cells (Fig. 5E). Thus, we find it likely that CD4 T cell cytotoxicity is mediated at least in part via mechanisms other than lytic granule release.
Figure 5. M2124-138-specific CD4 T cells are cytotoxic.
(A) Representative dot plots showing IFNγ production and CD107a expression by lung CD4 T cells at 12 or 35 d after WT γHV68 infection. Numbers in the plots indicate the percent of IFNγ+TNFα+ CD4 T cells expressing CD107a. (B) The percent of IFNγ+TNFα+ CD4 T cells that are CD107a+ in the lungs over time after WT γHV68 infection (n=5, representative of 4 experiments; ns, not significant; ***P≤0.001; one-way ANOVA). (C) Specific lysis of peptide-pulsed target cells (±SEM) in WT γHV68-infected mice (n=4–9, combined from 2 experiments). (D) Specific lysis of peptide-pulsed target cells (±SEM) in AC-RTA-infected mice (n=5–7, combined from 2 experiments). (E) 21 d after WT γHV68 infection spleens were harvested and analyzed by flow cytometry. (left) Representative histograms showing granzyme B (GzmB) expression in CD44hi or CD44lo CD4 (top) or CD8 T cells (bottom). (center) Representative dot plots showing expression of CD44 and either M2124-138/I-Ab (top) or ORF61524-531/Kb (bottom) tetramers. (right) The percent of cells expressing granzyme B. (n=7, representative of 3 experiments; ns, not significant; ***P≤0.001; one-way ANOVA).
Discussion
We have identified a panel of γHV68-specific CD4 epitopes and followed the expression of epitope-specific CD4 T cells throughout the course of natural γHV68 infection. Previously, we identified a panel of γHV68-specific CD8 T cell epitopes, and followed their expression (10). The combined results show that antiviral CD4 and CD8 T cells have very different kinetic patterns. Comparative analysis with WT virus and a recombinant virus incapable of establishing latency allowed us to examine the influence of latency on differential expression of epitope-specific responses. Whereas the CD8 T cells exhibited two basic patterns of expression apparently dependent on epitope expression during lytic and latent infection, the kinetics of CD4 T cells were more complex. One group of epitopes was expressed predominantly during the acute infection, a second group was expressed both during the acute infection and early stages of latency, and an additional epitope was expressed exclusively during latency. These data represent the first kinetic analysis of a panel of CD4 epitopes during γHV68 infection and illustrate the importance of latency in initiating and sustaining CD4 T cells specific for some but not all epitopes. We did not detect any evidence for inflation of the M2124-138-specific response (33), consistent with the temporally-restricted expression of the M2 gene (22).
The newly identified epitopes will facilitate analysis of antigen presentation during γHV68 infection. We recently demonstrated that B cells and DCs from latently-infected mice could each stimulate γHV68-specific CD4 T cells, but it is not yet known whether they present the same epitopes (34). Notably, EBV-specific cytolytic CD4 T cells recognize a variety of both lytic and latent epitopes on B cell lymphoma cells (35). Cytotoxic CD4 T cells specific for latent epitopes may be important because the majority of tumor cells sustain a latent infection whereas non-cytotoxic, cytokine-secreting lytic epitope-specific CD4 T cells may be crucial for targeting cells harboring reactivating virus to prevent full recrudescence. As CD4 T cells are capable of mediating protection in two models of γHV68-associated tumors (36, 37), it will be of particular interest to investigate the protective efficacy of latency-specific cytotoxic CD4 T cells in these systems.
In our hands, immunization with M2124-138 peptide in complete Freund’s adjuvant induced a strong cytokine-producing epitope-specific CD4 T cell response, but did not induce antiviral T cells that were protective from viral challenge or in reducing latent viral loads (data not shown). We believe this is at least in part due to a dearth of cytotoxic CD4 T cells generated after immunization, as our initial results showed little or no M2124-138-specific cytotoxicity (data not shown). Our data are consistent with the findings of Stevenson and colleagues who did not observe protection from challenge in an engineered latency epitope vaccination strategy even though they induced robust epitope-specific CD4 T cells (38). Previously, vaccination to induce gp15067-83-specific CD4 T cells has resulted in the generation of protective responses, although whether the T cells exhibited cytotoxicity is unclear (12).
Understanding how to induce and sustain a strong cytolytic CD4 T cell response by vaccination and how to generate CD4 effectors therapeutically are important challenges. It will be important to determine if other prophylactic vaccination strategies can induce virus-specific CD4 T cells capable of reducing acute infection or latency establishment, and whether therapeutic vaccination designed to boost M2-specific CD4 T cells reduces latent viral loads, thereby lessening the risk for oncogenesis. Of note, transferred naïve CD4 T cells have been shown to develop into anti-tumor effectors in vivo in at least two separate models (39, 40). Understanding the role of antiviral CD4 T cells in immune control of virus in this natural mouse infection model can provide important insight into mechanisms for tumor immunosurveillance in general.
Acknowledgments
We thank Eva Medina for excellent technical assistance, and Srividya Ramachandran and Lisa M. Connor for critically reading the manuscript. We gratefully acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC class II tetramer.
Footnotes
This work was supported by NIH grants F32AI084327 (MLF), T32AI049823 (DLW), CA168558 (LFvD), AI42927, AI082919, and CA148250 (MAB), NIH contracts HHSN272200900042C and HHSN272200900044C (AS), HHSN272201300006C (NIH Tetramer Core Facility), and funds from the Trudeau Institute.
Disclosure Statement
The authors have no financial conflicts of interest.
References
- 1.Stevenson PG, Efstathiou S, Doherty PC, Lehner PJ. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc Natl Acad Sci U S A. 2000;97:8455–8460. doi: 10.1073/pnas.150240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman MA, Flano E, Usherwood E, Woodland DL. Murine gamma-herpesvirus-68: a mouse model for infectious mononucleosis? Mol Med Today. 2000;6:488–490. doi: 10.1016/s1357-4310(00)01813-x. [DOI] [PubMed] [Google Scholar]
- 3.Martorelli D, Muraro E, Merlo A, Turrini R, Rosato A, Dolcetti R. Role of CD4+ cytotoxic T lymphocytes in the control of viral diseases and cancer. Int Rev Immunol. 2010;29:371–402. doi: 10.3109/08830185.2010.489658. [DOI] [PubMed] [Google Scholar]
- 4.Paludan C, Munz C. CD4+ T cell responses in the immune control against latent infection by Epstein-Barr virus. Curr Mol Med. 2003;3:341–347. doi: 10.2174/1566524033479771. [DOI] [PubMed] [Google Scholar]
- 5.Heller KN, Arrey F, Steinherz P, Portlock C, Chadburn A, Kelly K, Munz C. Patients with Epstein Barr virus-positive lymphomas have decreased CD4(+) T-cell responses to the viral nuclear antigen 1. Int J Cancer. 2008;123:2824–2831. doi: 10.1002/ijc.23845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Q, Freeman ML, Yager EJ, McHardy I, Tong L, Martinez-Guzman D, Rickabaugh T, Hwang S, Blackman MA, Sun R, Wu TT. Induction of protective immunity against murine gammaherpesvirus 68 infection in the absence of viral latency. J Virol. 2010;84:2453–2465. doi: 10.1128/JVI.01543-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. 2008;4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui HH, Sidney J, Peters B, Sathiamurthy M, Sinichi A, Purton KA, Mothe BR, Chisari FV, Watkins DI, Sette A. Automated generation and evaluation of specific MHC binding predictive tools: ARB matrix applications. Immunogenetics. 2005;57:304–314. doi: 10.1007/s00251-005-0798-y. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen M, Lundegaard C, Lund O. Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinformatics. 2007;8:238. doi: 10.1186/1471-2105-8-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman ML, Lanzer KG, Cookenham T, Peters B, Sidney J, Wu TT, Sun R, Woodland DL, Sette A, Blackman MA. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J Virol. 2010;84:2881–2892. doi: 10.1128/JVI.02229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winslow GM, Roberts AD, Blackman MA, Woodland DL. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J Immunol. 2003;170:2046–2052. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Usherwood EJ, Blackman MA, Woodland DL. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J Virol. 1999;73:9849–9857. doi: 10.1128/jvi.73.12.9849-9857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flano E, Woodland DL, Blackman MA, Doherty PC. Analysis of virus-specific CD4(+) t cells during long-term gammaherpesvirus infection. J Virol. 2001;75:7744–7748. doi: 10.1128/JVI.75.16.7744-7748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virgin HW, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arens R, Wang P, Sidney J, Loewendorf A, Sette A, Schoenberger SP, Peters B, Benedict CA. Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J Immunol. 2008;180:6472–6476. doi: 10.4049/jimmunol.180.10.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebrahimi B, Dutia BM, Brownstein DG, Nash AA. Murine gammaherpesvirus-68 infection causes multi-organ fibrosis and alters leukocyte trafficking in interferon-gamma receptor knockout mice. Am J Pathol. 2001;158:2117–2125. doi: 10.1016/s0002-9440(10)64683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long HM, Leese AM, Chagoury OL, Connerty SR, Quarcoopome J, Quinn LL, Shannon-Lowe C, Rickinson AB. Cytotoxic CD4+ T cell responses to EBV contrast with CD8 responses in breadth of lytic cycle antigen choice and in lytic cycle recognition. J Immunol. 2011;187:92–101. doi: 10.4049/jimmunol.1100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robey RC, Lagos D, Gratrix F, Henderson S, Matthews NC, Vart RJ, Bower M, Boshoff C, Gotch FM. The CD8 and CD4 T-cell response against Kaposi’s sarcoma-associated herpesvirus is skewed towards early and late lytic antigens. PLoS One. 2009;4:e5890. doi: 10.1371/journal.pone.0005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long HM, Chagoury OL, Leese AM, Ryan GB, James E, Morton LT, Abbott RJ, Sabbah S, Kwok W, Rickinson AB. MHC II tetramers visualize human CD4+ T cell responses to Epstein-Barr virus infection and demonstrate atypical kinetics of the nuclear antigen EBNA1 response. J Exp Med. 2013;210:933–949. doi: 10.1084/jem.20121437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gredmark-Russ S, Cheung EJ, Isaacson MK, Ploegh HL, Grotenbreg GM. The CD8 T-cell response against murine gammaherpesvirus 68 is directed toward a broad repertoire of epitopes from both early and late antigens. J Virol. 2008;82:12205–12212. doi: 10.1128/JVI.01463-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Flano E, Usherwood EJ, Surman S, Blackman MA, Woodland DL. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J Immunol. 1999;163:868–874. [PubMed] [Google Scholar]
- 22.Usherwood EJ, Roy DJ, Ward K, Surman SL, Dutia BM, Blackman MA, Stewart JP, Woodland DL. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J Exp Med. 2000;192:943–952. doi: 10.1084/jem.192.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virgin HW, Presti RM, Li XY, Liu C, Speck SH. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herskowitz JH, Jacoby MA, Speck SH. The murine gammaherpesvirus 68 M2 gene is required for efficient reactivation from latently infected B cells. J Virol. 2005;79:2261–2273. doi: 10.1128/JVI.79.4.2261-2273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby MA, Virgin HW, Speck SH. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J Virol. 2002;76:1790–1801. doi: 10.1128/JVI.76.4.1790-1801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macrae AI, Usherwood EJ, Husain SM, Flano E, Kim IJ, Woodland DL, Nash AA, Blackman MA, Sample JT, Stewart JP. Murid herpesvirus 4 strain 68 M2 protein is a B-cell-associated antigen important for latency but not lymphocytosis. J Virol. 2003;77:9700–9709. doi: 10.1128/JVI.77.17.9700-9709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen JP, Cardin RD, Branum KC, Doherty PC. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci U S A. 1999;96:5135–5140. doi: 10.1073/pnas.96.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuller KA, Cush SS, Flano E. Persistent gamma-herpesvirus infection induces a CD4 T cell response containing functionally distinct effector populations. J Immunol. 2010;184:3850–3856. doi: 10.4049/jimmunol.0902935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 31.Stuller KA, Flano E. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. J Virol. 2009;83:4700–4703. doi: 10.1128/JVI.02240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 33.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 34.Freeman ML, Burkum CE, Lanzer KG, Jensen MK, Ahmed M, Yager EJ, Flano E, Winslow GM, Woodland DL, Blackman MA. Cutting Edge: Activation of Virus-Specific CD4 T Cells throughout gamma-Herpesvirus Latency. J Immunol. 2011;187:6180–6184. doi: 10.4049/jimmunol.1102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heller KN, Gurer C, Munz C. Virus-specific CD4+ T cells: ready for direct attack. J Exp Med. 2006;203:805–808. doi: 10.1084/jem.20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson KA, Usherwood EJ, Nash AA. Regression of a murine gammaherpesvirus 68-positive b-cell lymphoma mediated by CD4 T lymphocytes. J Virol. 2001;75:3480–3482. doi: 10.1128/JVI.75.7.3480-3482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang X, Crepeau RL, Zhang W, Speck SH, Usherwood EJ. CD4 and CD8 T cells directly recognize murine gammaherpesvirus 68-immortalized cells and prevent tumor outgrowth. J Virol. 2013;87:6051–6054. doi: 10.1128/JVI.00375-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith CM, Rosa GT, May JS, Bennett NJ, Mount AM, Belz GT, Stevenson PG. CD4+ T cells specific for a model latency-associated antigen fail to control a gammaherpesvirus in vivo. Eur J Immunol. 2006;36:3186–3197. doi: 10.1002/eji.200636164. [DOI] [PubMed] [Google Scholar]
- 39.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackett M, Stewart JP, de VPS, Chee M, Efstathiou S, Nash AA, Arrand JR. Genetic content and preliminary transcriptional analysis of a representative region of murine gammaherpesvirus 68. J Gen Virol. 1997;78(Pt 6):1425–1433. doi: 10.1099/0022-1317-78-6-1425. [DOI] [PubMed] [Google Scholar]
- 42.Ahn JW, Powell KL, Kellam P, Alber DG. Gammaherpesvirus lytic gene expression as characterized by DNA array. J Virol. 2002;76:6244–6256. doi: 10.1128/JVI.76.12.6244-6256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng BY, Zhi J, Santana A, Khan S, Salinas E, Forrest JC, Zheng Y, Jaggi S, Leatherwood J, Krug LT. Tiled microarray identification of novel viral transcript structures and distinct transcriptional profiles during two modes of productive murine gammaherpesvirus 68 infection. J Virol. 2012;86:4340–4357. doi: 10.1128/JVI.05892-11. [DOI] [PMC free article] [PubMed] [Google Scholar]