Abstract
A glycoprotein osteopontin (OPN) is involved in inflammatory diseases, but its roles in inflammatory bowel disease (IBD) are controversial. To analyze the involvement of the systemic immune response, we simultaneously examined plasma OPN levels and 17 cytokines. This study included 24 ulcerative colitis (UC) patients, 17 Crohn's disease (CD) patients, and 23 normal controls. Clinical parameters were also examined. The plasma OPN levels of the UC and CD patients were significantly higher than those of the normal controls and correlated significantly with their clinical activity indices. In the UC patients, significant relationships were observed between the levels of plasma OPN and multiple cytokines, including interleukin (IL) −1β, IL-4, IL-5, IL-6, IL-7, IL-13, interferon-γ, tumor necrosis factor-α, and granulocyte-macrophage colony-stimulating factor. In the CD patients, the correlation was not significant except for IL-8. Our findings reflect different inflammatory states of the colon and rectum in both diseases.
Key words: Inflammatory bowel disease, Osteopontin, Ulcerative colitis, Crohn's disease, Cytokine
The etiologies of chronic inflammatory bowel disease (IBD), ulcerative colitis (UC), and Crohn's disease (CD) have not been fully elucidated. Although the etiology of IBD remains unknown, pathogenetic factors that have been implicated include microbial agents, immune dysfunction, genetic susceptibility and various environmental factors (such as diet).1 Of the multiple immunologic parameters, we have focused on osteopontin (OPN) as a novel clinical marker of IBD.2,3
OPN is a glycosylated phosphoprotein that plays an important role in bone metabolism, inflammation and immunologic function in response to infectious diseases.4,5 OPN is essential for Th1 immunity because it promotes interleukin-12 (IL-12) production by macrophages, increases the levels of interferon-γ (IFN-γ), and diminishes IL-10 levels in the lymph nodes drained from granulomatous tissues.6 There have been many reports suggesting the involvement of OPN in immune function. One report has demonstrated that experimental autoimmune encephalomyelitis in OPN-deficient OPN mice was milder than in wild-type mice.7 OPN-knockout mice exhibit milder symptoms associated with anti-type-II collagen antibody-induced experimental arthritis.8 Clinical reports have also suggested the possible importance of OPN in autoimmune diseases, on the basis of a positive correlation between plasma OPN levels and the neurologic activities of relapsing-remitting multiple sclerosis9 and a correlation between synovial fluid OPN levels and the local manifestations of rheumatoid arthritis.10
With respect to IBD, Gassler et al first demonstrated increased OPN expression in the terminal ilea of patients with CD.11 Significant correlations between plasma OPN levels and the disease activities of CD12,13 and UC14 have been reported.
In the context of UC, we reported a 23.4-fold increase in OPN mRNA expression compared with normal control colon tissues using gene-chip analysis.2 We confirmed these findings using protein-level analysis and localized macrophage-like cells in the colon using immunohistochemical techniques.3 However, the pathophysiological roles of plasma OPN and the relationships between other inflammatory markers and cytokine levels are, thus far, unknown. Here, we investigated the potential roles of OPN in the immune response to IBD by simultaneously examining multiple cytokines via suspension array analysis.
Materials and Methods
Patients and samples
Blood samples were obtained by venipuncture from patients with UC (n = 24), patients with CD (n = 17), and healthy volunteers (controls, n = 23) between November 2008 and November 2009. Inclusion criteria were the following: age > 18 years, both genders, and homogeneous ethnic group (Japanese). We excluded the patients who complicated with autoimmune diseases, diabetes mellitus, and local or systemic inflammations. Of the 24 patients with UC, 7 patients underwent proctocolectomy. The 17 UC patients who were not subjected to operations included 10 men and 7 women. Their ages ranged from 19 to 61 years old, with a mean of 41.8 years. There were 9 patients with a total colon type, 5 patients with left colitis, and 3 patients with proctitis. The 7 UC patients who underwent proctocolectomy included 4 men and 3 women (age range, 32−67 years; mean, 47.4 years). All 7 patients had total colitis-type IBD. The patients with CD consisted of 15 men and 2 women (age range, 18−51 years; average, 39.4 years). Of these 17 patients, 14 had the small-large intestinal type, where both the small and large intestines were affected. The healthy volunteers included 13 men and 10 women (age range, 25−53 years; average, 34.8 years). The clinical activities of the patients with UC were determined using the clinical activity index (CAI) and ulcerative colitis activity index (UCAI).15,16 For the patients with CD, Crohn's disease activity index (CDAI)17 and the index of inflammatory bowel disease (IOIBD)18 were determined. The ethics committee of Nihon University Nerima Hikarigaoka Hospital approved the study protocol. Written informed consent was obtained from the patients before sampling. The data obtained via routine laboratory examinations were also examined.
OPN
Plasma OPN levels were measured using an enzyme-based immunoassay with the Human Osteopontin Assay Kit (IBL, Takasaki, Gunma, Japan), according to the manufacturer's instructions. Briefly, 100 μL of osteopontin standards or plasma samples were added to 96-well plates coated with an anti-osteopontin antibody and incubated for 1 hour at 37°C. After incubation and washing, treatment with secondary antibody was carried out for 30 minutes at 4°C. After washing, plates were incubated with TMB substrate for 30 minutes. Reaction (color development) was stopped by adding 100 μL of 1% sodium dodecyl sulfate. Absorbance was read using a microplate reader at 450 nm.
Cytokines
Serum cytokine concentrations were measured using the Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA, USA) with the Bio-Plex Human 17-Plex Panel (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer's instructions. The Bio-Plex cytokine assay is designed for the multiplexed quantitative measurement of multiple cytokines in a single well using 50 μL of serum sample. In the present study, the premixed multiplex beads of the Bio-Plex Human 17-Plex Panel included 17 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, MCP-1, MIP-1β, and TNF-α). Briefly, 50 μL of cytokine standards or serum samples were incubated with 50 μL of anti-cytokine-conjugated beads in 96-well filter plates for 30 min at room temperature with shaking. The plates were washed three times with 100 μL of wash buffer by vacuum filtration, 25 μL of the diluted detection antibody was then added, and the plates were incubated for 30 minutes at room temperature with shaking. After three washes, 50 μL of streptavidin-phycoerythrin were added, and the plates were incubated for 10 minutes at room temperature with shaking. Finally, the plates were washed three times. The beads were suspended in Bio-Plex assay buffer and analyzed on a Bio-Rad 96-well plate reader using the Bio-Plex Suspension Array System and Bio-Plex Manager software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
The data were analyzed using Welch's t-test, Tukey-Kramer test, and Pearson's correlation coefficient test using Statcel 2 software (The Publisher OMS Ltd., Tokorozawa, Saitama, Japan). A probability level of 5% (P < 0.05) was considered to be statistically significant.
Results
Plasma OPN levels elevated in UC and CD patients
The plasma OPN levels of UC (380.3 ± 174.5 ng/mL) and CD patients (543.7 ± 316.7 ng/mL) were significantly higher than those of normal controls (254.7 ± 131.9 ng/mL; Fig. 1A). The plasma OPN levels of the seven UC patients managed by proctocolectomy were as low as normal control levels (269.2 ± 80.8 ng/mL; Fig.1B).
Fig. 1.
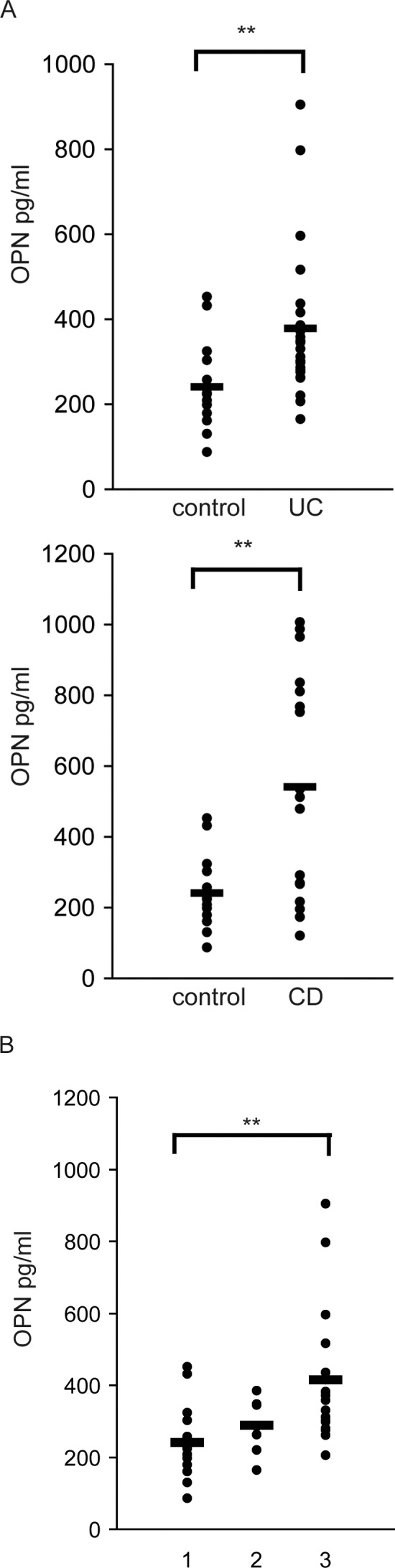
Plasma OPN levels were elevated both in patients with UC and CD. (A) The plasma OPN levels of UC and CD patients were significantly higher than those of normal controls. (B) In 7 UC patients managed via proctocolectomy, the plasma OPN levels after surgery were decreased compared with normal control levels. 1: control; 2: UC patients after proctocolectomy; 3: UC patients without surgical treatment. **, P < 0.01, as determined by Welch's t-test or Tukey-Kramer test.
Serum cytokine levels differentially elevated in IBD patients
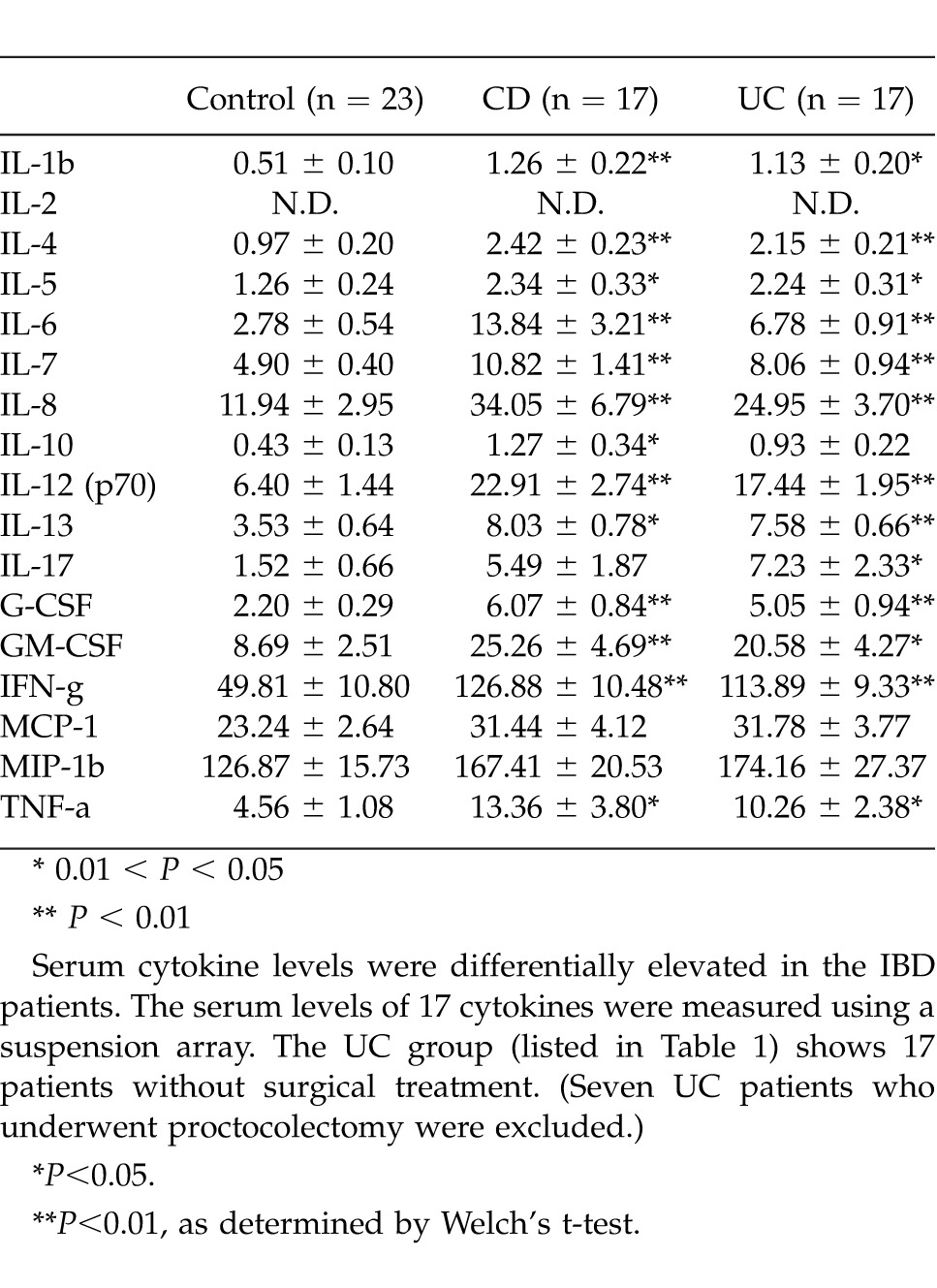
The 17 serum cytokine levels measured by the suspension array system are shown in Table 1. The UC group (listed in Table 1) shows the 17 patients without surgical treatment. (Seven UC patients who underwent proctocolectomy were excluded.) In the UC patients, all of the cytokines (except IL-10, MCP-1, and MIP-1β) were significantly elevated compared with normal controls. All of the cytokine levels in the CD group (except those of IL-17, MCP-1, and MIP-1β) were significantly greater than those of the normal control group. IL-2 was below the detection limit in both groups.
Table 1.
Serum cytokine level
Plasma OPN levels in IBD patients correlate with clinical indices and serum cytokine levels
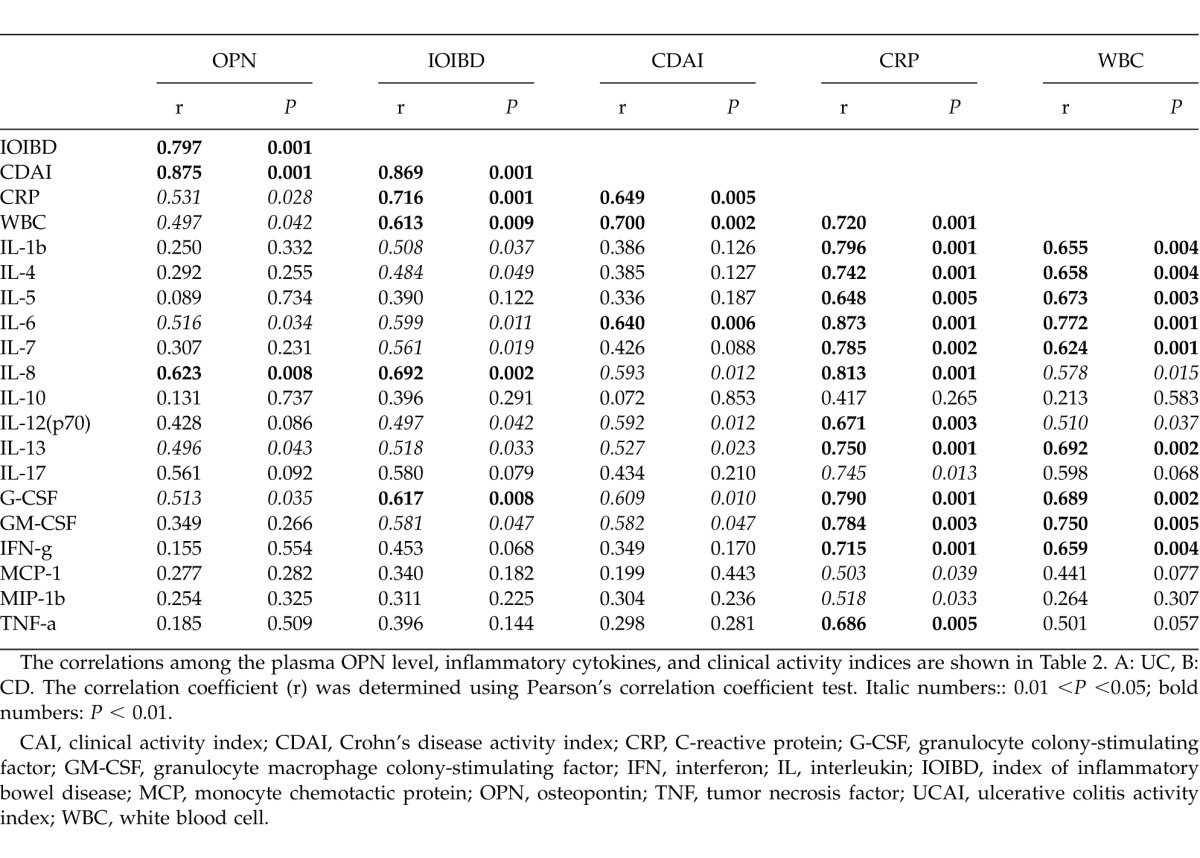
The correlations among the plasma OPN level, inflammatory cytokines and clinical activity indices are shown in Table 2. In both UC and CD, significant correlations were observed between the plasma OPN levels and clinical activity indices (Table 2A, B). However, correlation patterns between OPN and the other markers (including the serum cytokines) were dissimilar in these two diseases.
Table 2A.
Correlation of plasma OPN with clinical indices and serum cytokine levels (UC)
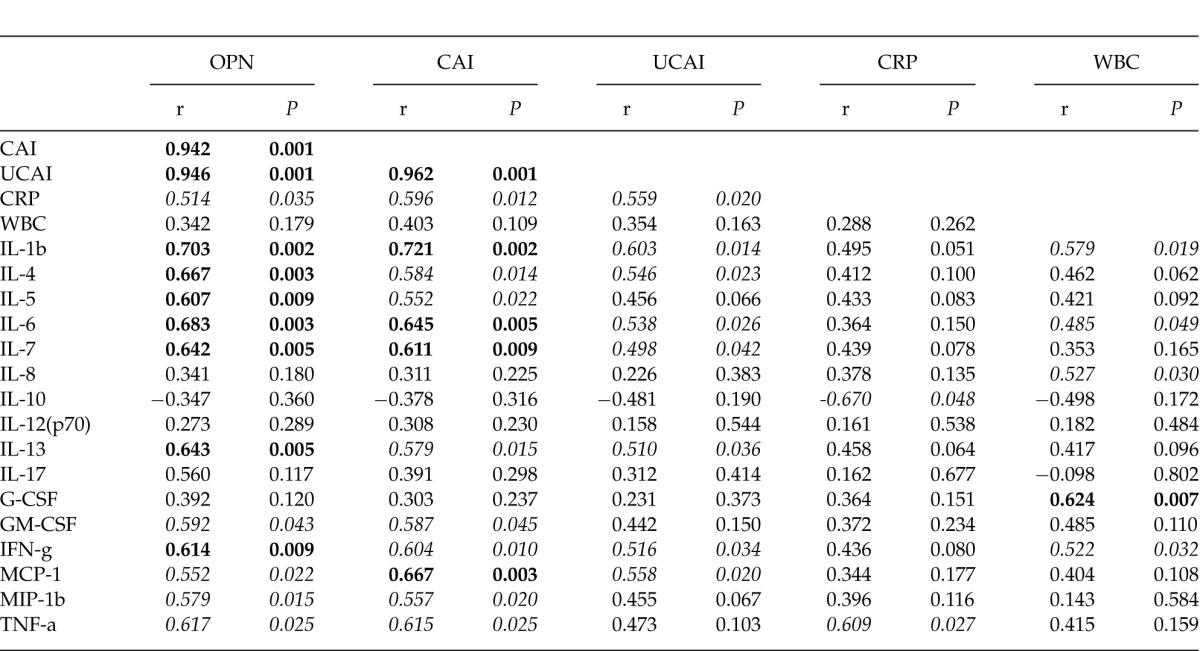
Table 2B.
Correlation of plasma OPN with clinical indices and serum cytokine levels (CD)
In the UC patients, remarkable correlations were observed between OPN and multiple inflammatory cytokines, including IL-1β, IL-4, IL-5, IL-6, IL-7, IL-13, and IFN-γ. In particular, the clinical activity indices (including the CAI and UCAI) had strong correlations with the inflammatory cytokines. However, weak correlations were observed between the CRP and WBC counts and other markers, including OPN and the clinical activity indices (Table 2A).
In the CD patients, we observed a remarkable correlation solely between OPN and IL-8. Interestingly, strong correlations were observed between the CRP and WBC counts and the inflammatory cytokines (Table 2B).
No correlations between OPN and inflammatory cytokines were found for the UC patients after proctocolectomy (data not shown).
Discussion
Idiopathic IBD consists of two forms, UC and CD. Although they share common backgrounds, they exhibit many clinical and pathologic differences. In the present study, we observed novel differences between these two diseases in terms of their associated plasma OPN levels, cytokines, and inflammatory markers. There have been many reports indicating a correlation among the clinical activity of IBD, serum cytokines and OPN levels. However, OPN levels and clinical activity of IBD are controversial. Sato et al reported elevated plasma OPN and significant correlation with disease activity in CD, but no correlation was reported for UC.12 However, Mishima et al reported a positive correlation between the serum OPN concentration and disease activity in UC.14 In the present study, OPN levels were increased in both diseases. In addition, we observed more significant relationships between OPN levels and clinical activity, as indicated by the CDAI, IOIBD, UCAI, and CAI. Interestingly, although the plasma levels of OPN were increased in CD, the relationship between the plasma OPN level and inflammatory cytokines was less prominent. However, the WBC counts and CRP levels correlated with the inflammatory cytokines. In UC, significant relationships were observed between the levels of many inflammatory cytokines and plasma OPN expression. Therefore, our findings suggest that the plasma OPN level more accurately reflects UC disease activity than general inflammatory markers, such as CRP levels and WBC counts.
Elevated serum IFN-γ, IL-6, TNF-α, IL-18, and IL-10 have been reported in the active phase of IBD.19,20 Although early reports have suggested CD and UC are Th1-and Th2-mediated inflammatory diseases, respectively,21 our results suggest that both the Th1 and Th2 cytokines are increased in both diseases. In this sense, we consider the respective classification of CD and UC as Th1- and Th2-mediated diseases to be an oversimplification.
Our results partially agree with those of previous publications,12,14 but only revealed significant relationships between OPN and inflammatory cytokines in UC. Furthermore, plasma OPN levels were reduced, and these relationships completely disappeared after proctocolectomy. This fact suggests that OPN is produced at the inflammation site and positively correlates with the pathophysiology of UC. We previously reported increased local production of OPN in inflamed bowel tissue at both the protein and mRNA levels.3 Here, we suggest possible roles for OPN in the disease process of UC and its use as a more reliable clinical marker of disease activity.
In conclusion, we propose the use of OPN as a clinical marker of UC and CD. Although there are many differences in the pathophysiologies of these two diseases, OPN reflects the inflammatory state of the colon and rectum.
Acknowledgments
This paper was supported by a Grant-in-Aid for Scientific Research. The authors report no disclaimers for this article.
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115(1):182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Masuda H, Takahashi Y, Asai S, Takayama T. Distinct gene expression of osteopontin in patients with ulcerative colitis. J Surg Res. 2003;111(1):85–90. doi: 10.1016/s0022-4804(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 3.Masuda H, Takahashi Y, Asai S, Hemmi A, Takayama T. Osteopontin expression in ulcerative colitis is distinctly different from that in Crohn's disease and diverticulitis. J Gastroenterol. 2005;40(4):409–413. doi: 10.1007/s00535-005-1567-2. [DOI] [PubMed] [Google Scholar]
- 4.Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl. 1998;30–31:92–102. [PubMed] [Google Scholar]
- 5.Patarca R, Saavedra RA, Cantor H. Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene. Crit Rev Immunol. 1993;13(3–4):225–246. [PubMed] [Google Scholar]
- 6.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 7.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168(5):2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 8.Yumoto K, Ishijima M, Rittling SR, Tsuji K, Tsuchiya Y, Kon S, et al. Osteopontin deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Proc Natl Acad Sci U S A. 2002;99(7):4556–4561. doi: 10.1073/pnas.052523599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt MH, Lopatinskaya L, Smits M, Polman CH, Nagelkerken L. Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann Neurol. 2003;53(6):819–822. doi: 10.1002/ana.10606. [DOI] [PubMed] [Google Scholar]
- 10.Ohshima S, Yamaguchi N, Nishioka K, Mima T, Ishii T, Umeshita-Sasai M, et al. Enhanced local production of osteopontin in rheumatoid joints. J Rheumatol. 2002;29(10):2061–2067. [PubMed] [Google Scholar]
- 11.Gassler N, Autschbach F, Gauer S, Bohn J, Sido B, Otto HF, et al. Expression of osteopontin (Eta-1) in Crohn disease of the terminal ileum. Scand J Gastroenterol. 2002;37(11):1286–1295. doi: 10.1080/003655202761020560. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A, et al. Osteopontin/Eta-1 upregulated in Crohn's disease regulates the Th1 immune response. Gut. 2005;54(9):1254–1262. doi: 10.1136/gut.2004.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnholt J, Kelsen J, Schack L, Hvas CL, Dahlerup JF, Sorensen ES. Osteopontin, a protein with cytokine-like properties, is associated with inflammation in Crohn's disease. Scand J Immunol. 2007;65(5):453–460. doi: 10.1111/j.1365-3083.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 14.Mishima R, Takeshima F, Sawai T, Ohba K, Ohnita K, Isomoto H, et al. High plasma osteopontin levels in patients with inflammatory bowel disease. J Clin Gastroenterol. 2007;41(2):167–172. doi: 10.1097/MCG.0b013e31802d6268. [DOI] [PubMed] [Google Scholar]
- 15.Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330(26):1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 16.Talstad I, Gjone E. The disease activity of ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1976;11(4):403–408. [PubMed] [Google Scholar]
- 17.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 18.de Dombal FT, Softley A. IOIBD report no 1: observer variation in calculating indices of severity and activity in Crohn's disease. International Organisation for the Study of Inflammatory Bowel Disease. Gut. 1987;28(4):474–481. doi: 10.1136/gut.28.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanai H, Iida T, Yamada M, Sato Y, Takeuchi K, Tanaka T, et al. Effects of adacolumn selective leukocytapheresis on plasma cytokines during active disease in patients with active ulcerative colitis. World J Gastroenterol. 2006;12(21):3393–3399. doi: 10.3748/wjg.v12.i21.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmud N, O'Connell MA, Stinson J, Goggins MG, Weir DG, Kelleher D. Tumour necrosis factor-alpha and microalbuminuria in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7(3):215–219. [PubMed] [Google Scholar]
- 21.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]


