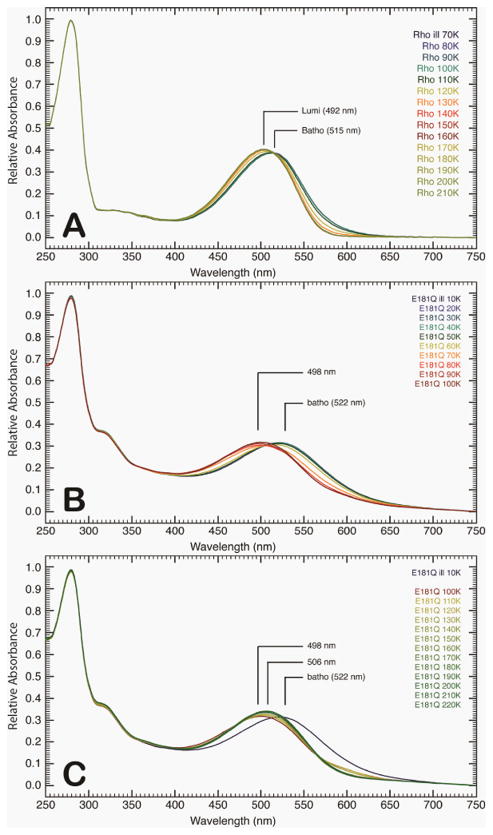
Figure 3.
Absorption spectra of rhodopsin (A) and E181Q (B and C) post-illumination as the temperature is ramped to 220 K via 10 K increments. The initial formation of the Batho photointermediate for native rhodopsin was achieved at 70 K (A), and as the temperature was increased, the formation of a single Lumi photointermediate (492 nm) was observed. The absorption spectra for E181Q was first collected at 10 K, in which the PSS522 consisted of a mixture of the dark and Batho states of the mutant protein (B). As the temperature was increased, a second PSS formed (PSS498) at 60 K. The E181Q sample was then allowed to warm to 220 K (C), where a third PSS (PSS506) evolved. The PSS498 was found to be a mixture of Batho and BSI, whereas the PSS506 was a Lumi photointermediate of the mutant protein (see text).