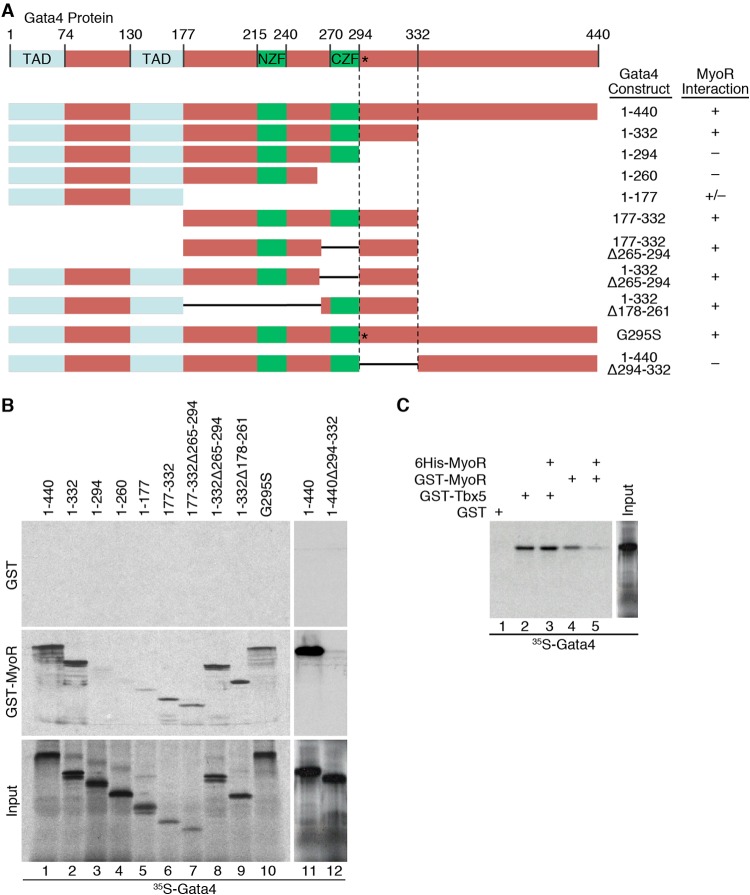
FIG 4.
MyoR interacts directly with Gata4 adjacent to the CZF and separate from the Tbx5 binding surface. (A) Schematic diagram of the Gata4 deletion proteins used in GST pulldown experiments with summarized MyoR interaction data. (B) In vitro-translated 35S-labeled Gata4 proteins were incubated with either GST alone (upper panel) or GST-MyoR (middle panel) to test for direct interaction. Input proteins are shown in the lower panel. Full-length Gata4 and deletions that preserve residues 294 to 332 interact directly with MyoR (lanes 1 and 2 and lanes 6 to 9). C-terminal deletion of Gata4 beyond amino acid 332 or internal deletion of residues 294 to 332 eliminates interaction with MyoR (lanes 3 to 5 and 12), showing that the region adjacent to the C-terminal zinc finger (CZF) is responsible for interacting with MyoR. In addition, the Gata4 G295S mutant retains the ability to interact with MyoR (lane 10), suggesting that MyoR and Tbx5 contact Gata4 via distinct binding surfaces. (C) GST pulldown competition assay with 35S-labeled Gata4 interacting with GST, GST-Tbx5, or GST-MyoR in the presence or absence of unlabeled His-tagged MyoR recombinant protein (lanes 1 to 5). Although unlabeled MyoR competes efficiently with GST-MyoR for Gata4 interaction (lanes 4 and 5), it is unable to prevent binding between GST-Tbx5 and Gata4 (lanes 2 and 3), providing additional evidence that the binding of Gata4 to MyoR or Tbx5 is not mutually exclusive. TAD, transactivation domain; NZF, N-terminal zinc finger; CZF, C-terminal zinc finger.