Figure 4.
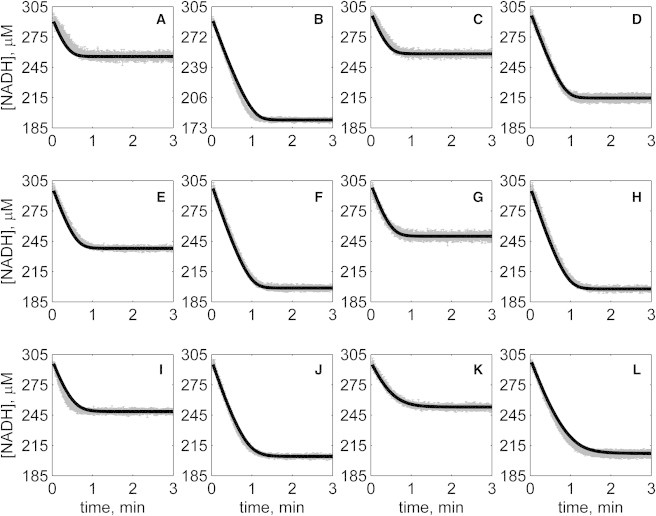
Progress curves of [NADH] versus time for reverse reaction (direction of NADH oxidation) at various pH values with 1 mM NAD as product inhibitor present in initial buffer. Initial conditions are [NADH]0 = 300 μM and (A) [OAA]0 = 50 μM, pH 6.5; (B) [OAA]0 = 100 μM, pH 6.5; (C) [OAA]0 = 50 μM, pH 7.0; (D) [OAA]0 = 100 μM, pH 7.0; (E) [OAA]0 = 50 μM, pH 7.5; (F) [OAA]0 = 100 μM, pH 7.5; (G) [OAA]0 = 50 μM, pH 8.0; (H) [OAA]0 = 100 μM, pH 8.0; (I) [OAA]0 = 50 μM, pH 8.5; (J) [OAA]0 = 100 μM, pH 8.5; (K) [OAA]0 = 50 μM, pH 9.0; and (L) [OAA]0 = 100 μM, pH 9.0. (In each plot the shaded lines and solid lines, respectively, represent mean with standard deviation of experimentally measured [NADH], and [NADH] obtained from fitting data to ordered bi-bi mechanism.)
