Table 3.
Adverse Events Associated With Treatment
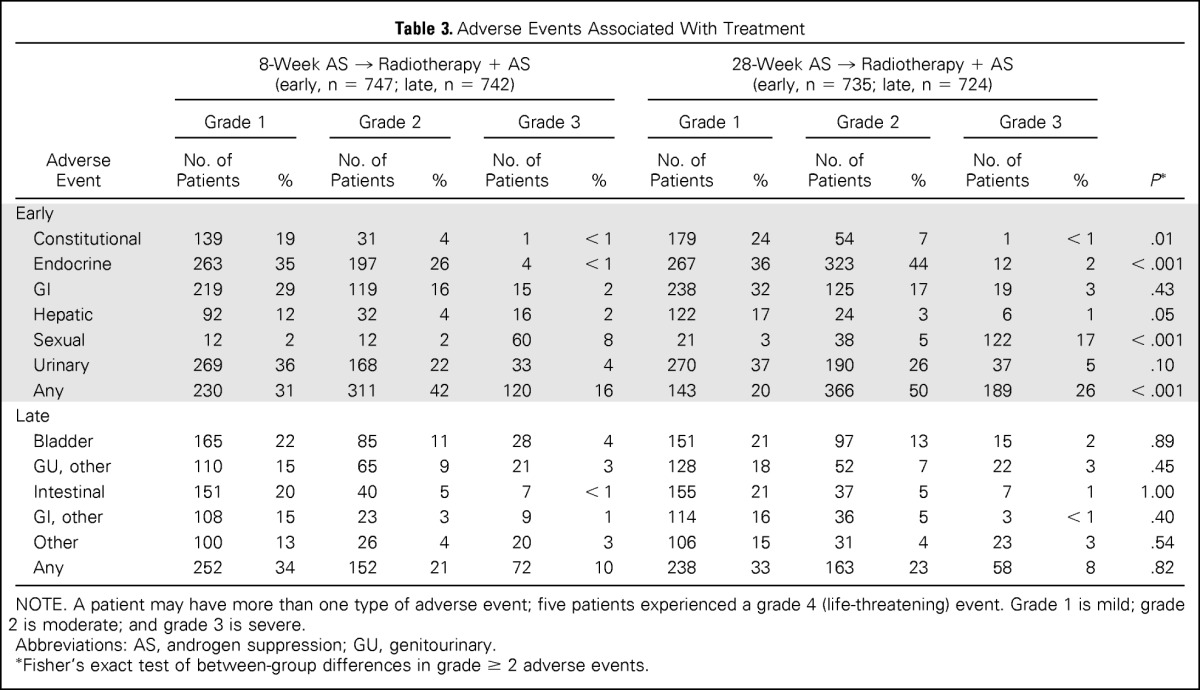
| Adverse Event | 8-Week AS → Radiotherapy + AS (early, n = 747; late, n = 742) |
28-Week AS → Radiotherapy + AS (early, n = 735; late, n = 724) |
P* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 1 |
Grade 2 |
Grade 3 |
||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Early | |||||||||||||
| Constitutional | 139 | 19 | 31 | 4 | 1 | < 1 | 179 | 24 | 54 | 7 | 1 | < 1 | .01 |
| Endocrine | 263 | 35 | 197 | 26 | 4 | < 1 | 267 | 36 | 323 | 44 | 12 | 2 | < .001 |
| GI | 219 | 29 | 119 | 16 | 15 | 2 | 238 | 32 | 125 | 17 | 19 | 3 | .43 |
| Hepatic | 92 | 12 | 32 | 4 | 16 | 2 | 122 | 17 | 24 | 3 | 6 | 1 | .05 |
| Sexual | 12 | 2 | 12 | 2 | 60 | 8 | 21 | 3 | 38 | 5 | 122 | 17 | < .001 |
| Urinary | 269 | 36 | 168 | 22 | 33 | 4 | 270 | 37 | 190 | 26 | 37 | 5 | .10 |
| Any | 230 | 31 | 311 | 42 | 120 | 16 | 143 | 20 | 366 | 50 | 189 | 26 | < .001 |
| Late | |||||||||||||
| Bladder | 165 | 22 | 85 | 11 | 28 | 4 | 151 | 21 | 97 | 13 | 15 | 2 | .89 |
| GU, other | 110 | 15 | 65 | 9 | 21 | 3 | 128 | 18 | 52 | 7 | 22 | 3 | .45 |
| Intestinal | 151 | 20 | 40 | 5 | 7 | < 1 | 155 | 21 | 37 | 5 | 7 | 1 | 1.00 |
| GI, other | 108 | 15 | 23 | 3 | 9 | 1 | 114 | 16 | 36 | 5 | 3 | < 1 | .40 |
| Other | 100 | 13 | 26 | 4 | 20 | 3 | 106 | 15 | 31 | 4 | 23 | 3 | .54 |
| Any | 252 | 34 | 152 | 21 | 72 | 10 | 238 | 33 | 163 | 23 | 58 | 8 | .82 |
NOTE. A patient may have more than one type of adverse event; five patients experienced a grade 4 (life-threatening) event. Grade 1 is mild; grade 2 is moderate; and grade 3 is severe.
Abbreviations: AS, androgen suppression; GU, genitourinary.
Fisher's exact test of between-group differences in grade ≥ 2 adverse events.
