Fig. 1.
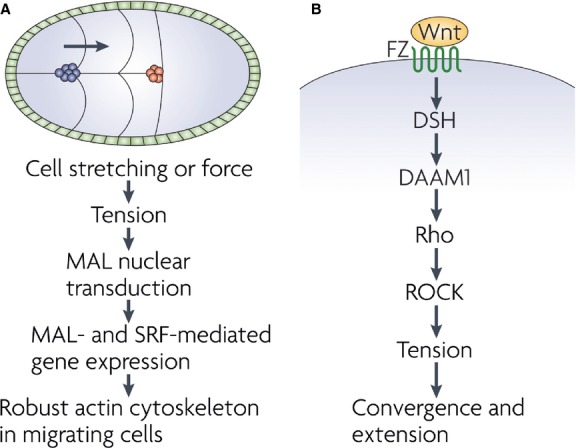
(A) During the border cell migration process of Drosophila melanogaster oogenesis, follicle cell migration proceeds along the midline of the egg chamber (the cells in blue are the migrating cells, and the cells in red indicate their final positions). The tension created as these cells are stretched or subjected to external force during their migration causes nuclear translocation of MAL, the SRF cofactor. As a result, the nuclear MAL and SRF are able to regulate the expression of various genes, including those needed for cytoskeletal integrity. The illustrated model is suggested to explain how cells form and maintain a strong actin cytoskeleton during their migration. (B) The planar cell polarity pathway, which is also called the non-canonical Wnt pathway, regulates various morphogenetic movements that result in spatial cell and tissue rearrangements during extension and convergence. When the Frizzled (Fz) receptor is binded by Wnt, Disheveled (Dsh) is then activated, which in turn activates Daam1, which in turn results in RhoA activation and ROCK-generated cellular tension and contractility. (Figure adapted from Wozniak M, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009; 10: 34–43.)
