Figure 1.
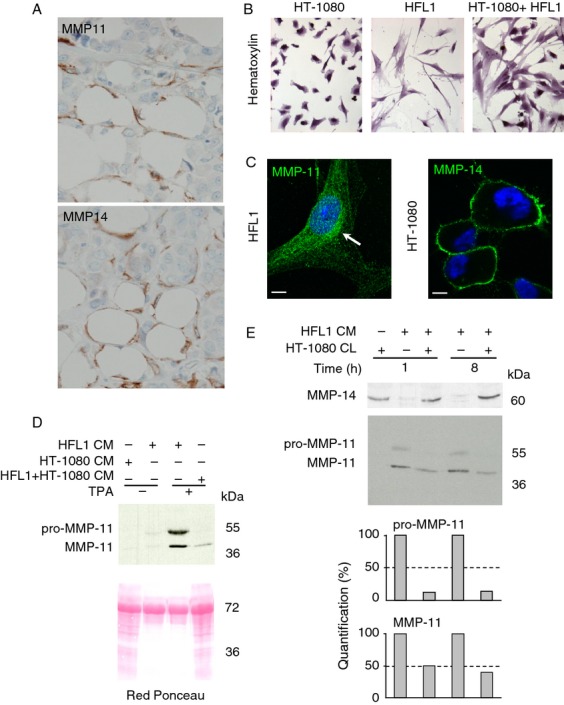
In vivo and in vitro MMP-11 and MMP-14 expression. (A) MMP-11 and MMP-14 immunohistochemistry of serial sections from an human invasive front of breast carcinoma using 5ST4A9 and 3H3 antibodies, respectively. Both MMPs (brown) were present in fibroblasts and CAAs (lipids in white) located adjacent to invading cancer cells showing that, in vivo, MMP-11 and MMP-14 are close proteins. Nuclei were in blue. Original magnification: 400×. (B) Hematoxylin staining of HT-1080 and HFL1 cells grown either alone or together. (C) Immunofluorescence analyses visualized MMP-14 at the HT-1080 cell membrane (green) and MMP-11 in the HFL1 secretory pathway (green, white arrow). Nuclei are in blue. Bars: 2 microns. (D) Coculture of the two cell lines led to a dramatic decreased levels of MMP-11 in the CM. Red Ponceau stained the secreted proteins. (E) Western blot of HFL1 CM (10 μg protein total containing HFL1-produced MMP-11) and HT-1080 CL (90 μg protein total containing HT-1080-produced MMP-14) incubation mixtures (1 and 8 h, RT). More than 50% of the pro-MMP-11 and 50% of MMP-11 were hydrolyzed after 1 h. MMP-14 remained similar in the presence or in the absence of HFL1 CM and whatever the MMP-11 levels, indicating that MMP-11 does not cleave MMP-14. Data from one representative experiment. CAA, cancer-associated adipocytes; CM, conditioned media; CL, cell lysates; MMP-14, matrix metalloproteinase-14.
