Figure 2.
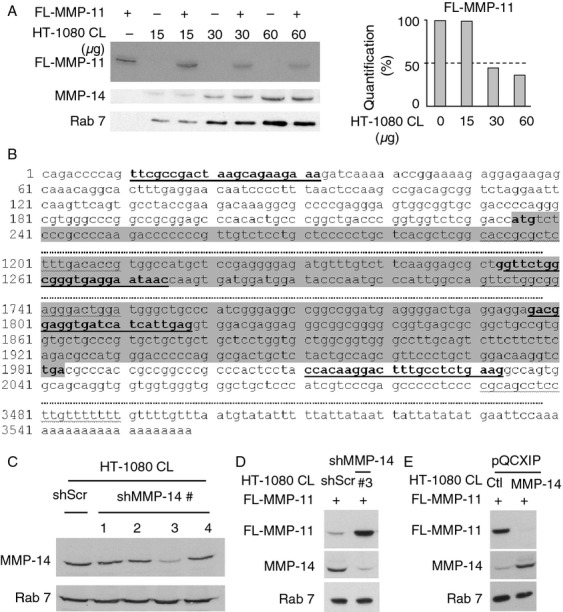
Analysis of cleavage of recombinant FL-MMP-11 by HT-1080-produced MMP-14. (A) Western blot of FL-MMP-11 (90 nmol/L) incubated with HT-1080 CL (15–60 μg protein total) showed a dose-dependent decrease in FL-MMP-11 intensity, indicating that HT-1080 CL has enzymatic activity toward MMP-11. No MMP-11 was present in HT-1080 CL. MMP-14 levels remained constant whatever the FL-MMP-11 status, indicating that MMP-11 does not cleave MMP-14. (B) The four putative MMP-14 shRNA target sequences of human MMP-14 mRNA (underlined, #1 to #4 5′–3′); ATG and TGA are in bold, coding sequence in grey. (C) CL Western blot showed that MMP-14 was dramatically reduced in HT-1080/shMMP-14#3 cell pool. (D) FL-MMP-11 (90 nmol/L) incubation with HT-1080/shScr CL (90 μg protein total; 2 h; RT) led to its decrease but not with HT-1080/shMMP-14#3 CL. (E) FL-MMP-11 (90 nmol/L) incubation with HT-1080/pQCXIP-MMP-14 CL (90 μg protein total; 2 h; RT) decreased FL-MMP-11 level compared with HT-1080/pQCXIP CL. Thus, FL-MMP-11 cleavage is MMP-14 dependent. Rab 7 serves as loading control. (A and C–E) Data from one representative experiment. CL, cell lysates. MMP-14, matrix metalloproteinase-14.
