Figure 3.
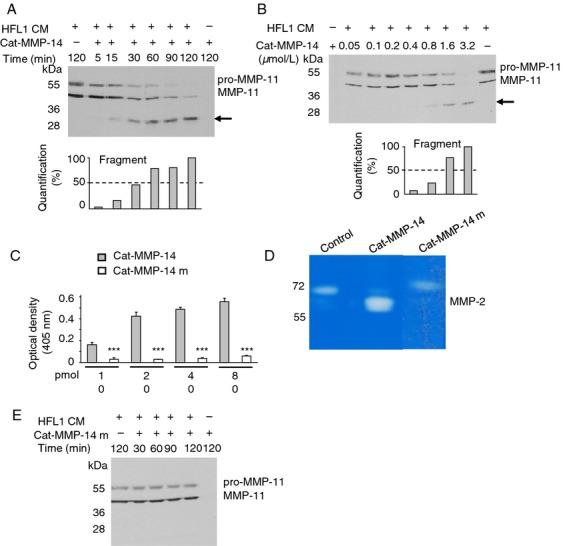
Analysis of cleavage of HFL1-produced MMP-11 by recombinant Cat-MMP-14. (A) HFL1 CM (10 μg protein total) were incubated with buffer alone or with Cat-MMP-14 (6.5 μmol/L) for increasing times (5–120 min; RT). Mixtures were electrophoresed (10% SDS-PAGE). Western blotting visualized the pro-MMP-11 and MMP-11 bands. One additional lower band appeared after 5 min and rose 50% of intensity around 45 min incubation (black arrow). Concomitantly, the 2 MMP-11 bands progressively disappeared. (B) Increasing amounts of Cat-MMP-14 (0.05–3.2 μmol/L) incubated with constant HFL1 CM quantity (10 μg protein total; 2 h; RT) showed lower band appearance and increase from 0.4 to 3.2 μmol/L of Cat-MMP-14 (black arrow), whereas pro-MMP-11 and MMP-11 disappeared. (C) Mutated Cat-MMP-14 m had no enzymatic activity toward a1-PI compared with the active Cat-MMP-14 (P < 0.001). (D) cat-MMP-14 (0.5 μg) and cat-MMP-14 m (0.5 μg) activity were assayed by zymography. U87MG-produced pro-MMP-2 was activated by cat-MMP-14, whereas cat-MMP-14 m had no effect compared with the control (no MMP-14 incubation). (E) In experimental conditions similar to (A), Cat-MMP-14 m had no effect. (A–E) Data from one representative experiment. HFL1, human fetal lung fibroblast. CM, conditioned media.
