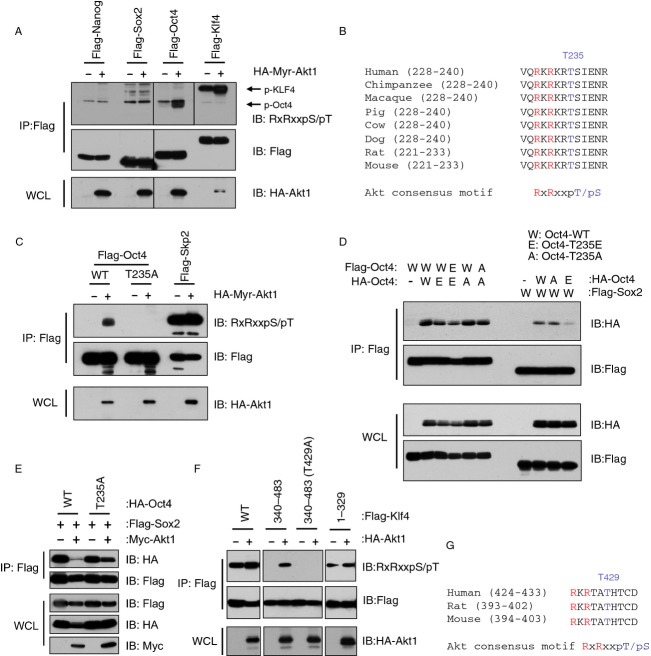
Figure 1.
Oct4 and Klf4 are phosphorylated by Akt in vivo. (A) Immunoblot (IB) analysis of whole cell lysates (WCLs) and immunoprecipitates (IPs) derived from 293T cells transfected with HA-tagged Myr-Akt1 and indicated Flag-tagged constructs. Akt-mediated phosphorylation was recognized by an Akt substrate-motif phosphorylation-specific antibody (RxRxxpS/pT). (B) Sequence alignment of the Thr235 putative Akt phosphorylation site in Oct4 among different species. (C) Akt specifically phosphorylated Oct4 at Thr235. (D) Phosphomimetic mutation at Thr235 of Oct4 decreased Oct4 interaction with Sox2. IB analysis and Flag-IP derived from 293T cells transfected with indicated constructs. (E) Phosphorylation of Oct4 on Thr235 led to attenuated Oct4 interaction with Sox2. WCLs of 293T cells transfected with indicated constructs were subjected to immunoprecipitation with Flag antibody. The Flag-IPs and WCLs were immunoblotted with indicated antibodies. (F) Akt phosphorylated Klf4 at Thr399. WCLs of 293T cells transfected with indicated constructs were subjected to immunoprecipitation with Flag antibody. The Flag-IPs and WCLs were immunoblotted with indicated antibodies. (G) Sequence alignment of the Thr429 putative Akt phosphorylation site in Klf4 among different species.