Abstract
Background
The incidence of melanoma, one of the most aggressive of the skin cancers, has been increasing worldwide in the last few decades. Data from Latin America and Brazil remain scarce. We aimed to describe the demographic, clinical, and histopathological data; therapy characteristics; and survival rates of the Brazilian melanoma patient population.
Results
We collected and analysed retrospective data from 15 years at a tertiary cancer centre. We describe patient characteristics and treatment. We calculated survival, and identified the main prognostic factors through univariate and multivariate analysis. We analysed a total of 1073 patients, with a mean age of 56.7 years. Men and women experienced similar prevalence, and 91.2% of patients had white skin. The most prevalent subtype was superficial spreading, and the most prevalent anatomic location was the trunk (32.2%), followed by the lower extremities (28%). Of all cases, 567 (52.9%) were assigned to clinical stages I and II, while 382 (32.6%) were stages III and IV. Surgery was the main treatment. Sentinel node biopsy was performed in 373 patients, with 23.8% positivity. Overall actuarial 5-year survival was 67.6%. Multivariate analysis showed that gender, serum lactate dehydrogenase (LDH) levels at diagnosis; anatomic location, TNM stage, and local recurrence were significant prognostic factors.
Conclusions
Overall survival was lower than worldwide rates. The main factors influencing survival were similar to those in other populations. Local recurrence was independently associated with lower survival rates. The high prevalence of advanced cases reinforces the importance of strategies to diagnose melanomas in the early stages. There is a need for future multi-institutional prospective studies to attain a better understanding of possible socioeconomic and other influences on survival among melanoma populations in Brazil and Latin America.
Keywords: Brazil, Melanoma, Cutaneous malignant, Latin America, Prognosis, Sentinel lymph node biopsy, Skin neoplasms
Background
Melanoma is a neoplasm arising from melanocytes [1] in the skin, mucosa, or uvea. Melanoma constitutes less than 5% of skin cancers but is responsible for around 95% of skin cancer deaths, and its incidence has been rising worldwide over recent decades [2]. In the United States, approximately 70,000 cases are diagnosed every year, with approximately 9000 deaths. In Brazil, it is estimated that 6000 new cases occur each year, resulting in 1300 deaths [3-5]. Risk factors for melanoma development include exposure to ultraviolet (UV) rays, as well as individual phenotypical characteristics, such as fair skin/hair pigmentation, presence of multiple nevi, immunosuppression, and family history [6].
Melanoma prognosis is based on clinical and histopathological factors. For localized disease, the primary tumour thickness (Breslow) is the most important prognostic factor. Tumour ulceration, mitotic rate, gender, age, and serum levels of lactate dehydrogenase (LDH) are also related to prognosis [7-10]. For metastatic disease, the presence and characteristics of lymph nodes and distant metastases are the major factors impacting survival. Lymph node tumour burden and the diagnosis of micrometastases by sentinel node biopsy (SNB) have been shown to be closely associated with prognosis. Although SNB is not intended as therapy, its results often change therapeutic plans. In standard practice, patients with positive SNBs, as well as patients with clinically diagnosed stage III disease, undergo lymph node dissection [11-14].
The different characteristics of melanoma and their relation to prognosis have been extensively reviewed [7,8,10,15]. In 2001, melanoma became the first cancer to be staged using an evidence-based system created on an extensive multi-institutional database (more than 17,000 melanoma cases) by the American Joint Committee on Cancer and the International Union Against Cancer (UICC) [8]. Unfortunately, this database included no Latin American or Brazilian patients. Skin phenotype and sun exposure are factors associated with melanoma incidence, while early diagnosis and easy access to healthcare are related to the prognosis. The Brazilian population is made up of multiple races, with many people of more than one racial heritage, and, hence, a range of skin colours. The population is frequently exposed to UV radiation. Access to healthcare is often difficult, especially for those living in rural areas. The Latin America and/or Brazilian published data are limited to descriptive studies or related to treatment in small series, and none has investigated survival or prognosis [16].
We sought to characterize Brazilian melanoma patients according to demographic, clinical, and histological data, as well as treatment, and to analyse the main factors related to prognosis.
Results
The study included 1073 patients, whose demographic characteristics are presented in Table 1. The main clinical and histopathological features are shown in Table 2 and treatment characteristics in Table 3. Sao Paulo State was the origin of 781 (72.8%) patients, followed by Minas Gerais with 128 (11.9%). The more distant areas—the North, Northeast, and Central Brazil area—accounted 151 cases (15%). From Sao Paulo State, 160 patients were from the Barretos area (20.5%) and 621 (79.5%) from other areas. The anatomical and histological subtype distributions of primary tumours differed according to skin colour characteristics. Compared with patients with white skin, a higher proportion of those with non-white skin presented with melanomas in the lower extremities (27.9 versus 54.1% p < 0.001) and more of the acral subtype (5.6 versus 18.5% p = 0.002). Of 34 non-white patients with tumours in the lower extremities, 10 presented with acral lentiginous melanomas (29.4%), 11 with nodular (32.4%), seven with superficial spreading (20.6%), one with lentigo maligna (2.9%), and five other/not otherwise specified (14.7). Women presented with more melanomas in the lower extremities than men (36.3 versus 23.2%; p < 0.001), and fewer in the trunk (29 versus 40%; p < 0.001). The superficial spreading histologic subtype was generally more prevalent among women (43.6 versus 33.7%; p = 0.035), while the nodular subtype was more common among men (34.6 versus 28.5%; p = 0.035).
Table 1.
Demographics of 1073 melanoma patients
| Patient characteristics | |
|---|---|
| Gender, n (%) | |
| Male | 543 (50.6) |
| Female | 530 (49.4) |
| Skin colour, n (%) | |
| White | 978 (91.2) |
| Other | 87 (8.1) |
| Not available | 8 (0.7) |
| Age, range (mean, SD) | 1-95 (56.7, 16.0) |
Table 2.
Main clinical and histological characteristics of 1073 melanoma patients
| Characteristics | Number | % |
|---|---|---|
| Anatomical location (primary tumour) | ||
| Trunk | 347 | 32.2 |
| Lower extremities | 300 | 28.0 |
| Head and neck | 197 | 18.4 |
| Upper extremities | 149 | 13.9 |
| Mucosa | 3 | 0.3 |
| Multiple | 2 | 0.2 |
| Unknown | 9 | 0.8 |
| Not available | 66 | 6.2 |
| Histological subtype | ||
| Superficial spreading | 353 | 32.9 |
| Nodular | 288 | 26.8 |
| Acral lentiginous | 76 | 7.1 |
| Lentigo maligna melanoma | 40 | 3.7 |
| Other/not classified | 156 | 14.6 |
| Not available | 160 | 14.9 |
| Tumour depth (Breslow) | ||
| Up to 1.0 mm | 225 | 21.0 |
| 1.1 to 2.0 mm | 184 | 17.1 |
| 2.1 to 4.0 mm | 171 | 15.9 |
| More than 4.0 mm | 231 | 21.5 |
| Not available | 262 | 24.4 |
| Clark classification | ||
| II | 110 | 10.3 |
| III | 269 | 25.1 |
| IV | 350 | 32.6 |
| V | 129 | 12.0 |
| Not available | 216 | 20.0 |
| Ulceration | ||
| Present | 307 | 28.6 |
| Absent | 371 | 34.6 |
| Not available | 395 | 36.8 |
| Mitotic index | ||
| Up to 1 mitosis/mm2 | 104 | 9.7 |
| More than 1 mitosis/mm2 | 260 | 24.2 |
| Not available | 709 | 66.1 |
| Lymph node status | ||
| N0 | 615 | 57.3 |
| N1 | 87 | 8.1 |
| N2 | 72 | 6.6 |
| N3 | 87 | 8.1 |
| Not available | 212 | 19.8 |
| Clinical stage (AJCC) | ||
| I | 296 | 27.6 |
| II | 271 | 25.3 |
| III | 214 | 19.9 |
| IV | 168 | 15.7 |
| Not available | 124 | 11.6 |
Table 3.
Treatment characteristics of 1073 melanoma patients
| Treatment | n | % |
|---|---|---|
| Surgery (primary tumour) | ||
| Not operated | 135 | 12.6 |
| Primary closure | 385 | 35.8 |
| Local surgery* | 257 | 20 |
| Local surgery and reconstruction (graft or flap) | 213 | 23.8 |
| Amputation | 64 | 6.0 |
| Second intention healing | 19 | 1.8 |
| Sentinel node biopsy | ||
| No | 700 | 73.8 |
| Yes | 373 | 26.2 |
| Positive | 89 | 23.9 |
| Negative | 251 | 76.3 |
| Not accessed/available | 33 | 8.8 |
| Adjuvant therapy | ||
| No | 908 | 84.6 |
| Radiation | 37 | 3.4 |
| Systemic | 86 | 8.0 |
| Not Available | 42 | 3.9 |
| Systemic therapy | ||
| No | 471 | 43.9 |
| Yes | 180 | 16.7 |
| Not available | 422 | 39.3 |
| Radiation therapy (metastasis) | ||
| No | 518 | 48.3 |
| Yes | 133 | 12.4 |
| Not available | 422 | 39.3 |
*Reconstructive surgery information not available.
In accordance with international treatment guidelines, surgery was the most common treatment modality, with a few patients receiving adjuvant radiation or systemic therapy. Amputation was mainly used to treat tumours in fingers/toes and advanced tumours of the extremities; no amputations were necessary to treat complications. Of the 135 patients not operated on for the primary tumour, 95 had received previous treatment at other institutions and 60 cases were stage IV; 33 were stage III or featured nodal recurrence. Twenty-nine patients underwent surgery at our institution. Twenty-seven patients received no treatment. These were advanced cases and/or refused treatment. In 373 cases, sentinel node biopsy (SNB) was performed using preoperative dynamic lymphoscintigraphy with 99mTc-labelled sulphur colloid and intraoperative blue dye and a gamma probe [17]. The rate of positivity was 23.8% (89 patients). Thirty-three cases (8.8%) had technical problems, or data were unavailable from the referring site. Among the 74 positive sentinel node cases with known tumour thickness (pT), only four cases (6.3%) were ≤ 1 mm (pT1), 16 (17.2%) were pT2, 18 (23.4%) were pT3, and 36 (41.9%) were pT4 (Table 4). Of the 284 negative cases (76.2%), locoregional recurrence occurred in 28 (9.8%).
Table 4.
Tumour thickness in patients submitted to sentinel node biopsy
| Tumour thickness (pT) | Total | Positive | Negative |
|---|---|---|---|
| pT1, n (%) | 63 (16.9) | 4 (6.4) | 59 (93.6) |
| pT2, n (%) | 93 (24.9) | 16 (17.2) | 77 (82.8) |
| pT3, n (%) | 77 (20.6) | 18 (23.4) | 59 (76.6) |
| pT4, n (%) | 86 (23.0) | 36 (41.9) | 50 (58.1) |
| Not available, n (%) | 54 (14.5) | 15 (27.8) | 39 (72.2) |
| Thickness in mm, mean (SD) | 3.3 (3.2) | 5.0 (4.4) | 2.7 (2.4) |
Survival analysis included 915 patients. At the last evaluation, 513 (56.1%) were alive and disease-free, 46 (5.0%) were alive with cancer, 266 (29.1%) had died due to melanoma, 79 (8.6%) had died from other causes, six (0.7%) died due to unknown causes, and five (0.5%) were lost to follow-up. Follow-up duration ranged from 6 to 233 months (mean 52 [SD 45] median, 38 months). For all patients alive at the last evaluation (559 cases), the mean follow-up was 63.5 months (SD 45), median 62 months. In 61.2% follow-up lasted longer than 3 years. Only five patients were lost to follow-up (0.5%). Among all patients, the 5-year disease-specific survival (DSS) rate was 67.6% (Figure 1).
Figure 1.
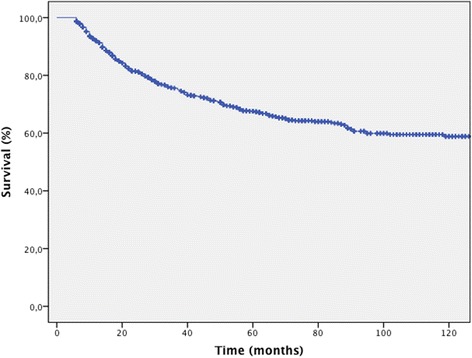
Cancer-specific survival for all melanoma patients.
Univariate analysis showed that the following demographic, clinical, and histological variables were related to prognosis: age (p = 0.005; Hazard Ratio = 1.011), gender, location of primary tumour, sentinel node positivity, Clark level, histologic subtype, presence of mitosis, ulceration, and microscopic satellitosis. Vascular and/or lymphatic infiltration were not statistically significant predictors of survival (Tables 5 and 6).
Table 5.
Disease-specific survival (DSS) according to demographic categories
| Variable | Categories | n | 5-year DSS (%) | p |
|---|---|---|---|---|
| Gender | Male | 448 | 59.1 | <0.001 |
| Female | 467 | 75.6 | ||
| Skin colour | White | 840 | 67.8 | 0.784 |
| Other | 68 | 69.3 |
Table 6.
Disease-specific survival (DSS) according to clinical and histological characteristics
| Variable | Category | n | 5-year SCS (%) | p |
|---|---|---|---|---|
| Anatomical location | Upper limbs | 139 | 75.9 | <0.001 |
| Trunk | 303 | 72.0 | ||
| Head and neck | 172 | 65.3 | ||
| Lower limbs | 264 | 62.1 | ||
| Unknown | 7 | 50.0 | ||
| Sentinel node | Negative | 258 | 80.6 | <0.001 |
| Positive | 77 | 54.9 | ||
| Clark | II | 97 | 93.4 | <0.001 |
| III | 246 | 80.2 | ||
| IV | 313 | 67.7 | ||
| V | 106 | 38.3 | ||
| Histological subtype | Acral lentiginous | 53 | 50.5 | <0.001 |
| Nodular | 258 | 61.3 | ||
| Superficial Spreading | 326 | 82.3 | ||
| Lentigo maligna | 34 | 87.5 | ||
| Not classified/other | 136 | 86.4 | ||
| Mitosis/mm2 | 0-1 | 95 | 77.5 | 0.005 |
| >1 | 236 | 62.2 | ||
| Intratumoural lymphocyte infiltration | Yes | 227 | 63.8 | 0.224 |
| No | 228 | 71.3 | ||
| Peritumoural lymphocyte infiltration | Yes | 366 | 71.3 | 0.136 |
| No | 162 | 57.4 | ||
| Vascular and lymphatic infiltration | Yes | 29 | 50.0 | 0.066 |
| No | 321 | 68.5 | ||
| Perineural invasion | Yes | 14 | 68.8 | 0.974 |
| No | 303 | 67.9 | ||
| Ulceration | Present | 260 | 56.8 | <0.001 |
| Absent | 332 | 78.0 | ||
| Regression | Yes | 57 | 73.3 | 0.863 |
| No | 413 | 69.2 | ||
| Microscopic satellitosis | Yes | 44 | 37.2 | <0.001 |
| No | 403 | 74.0 |
According to clinical stage and recurrence status, all TNM variables, presence of locoregional or distant recurrence, and serum LDH levels were associated with prognosis (Table 7). Women had higher survival rates than men (Table 5). TNM stage distribution also differed according to sex, with a higher percentage of women with lower stage disease than men (Table 8).
Table 7.
Disease-specific survival (DSS) according to clinical stage and recurrence status
| Variable | Category | n | 5-year DSS (%) | p |
|---|---|---|---|---|
| T | T1 | 203 | 93.1 | <0.001 |
| T2 | 175 | 75.9 | ||
| T3 | 162 | 59.6 | ||
| T4 | 204 | 53.1 | ||
| N | N0 | 577 | 80.3 | < 0.001 |
| N1 | 81 | 52.3 | ||
| N2 | 63 | 43.3 | ||
| N3 | 78 | 27.8 | ||
| M | M0 | 732 | 72.4 | < 0.001 |
| M1 | 105 | 26.1 | ||
| Clinical stage (TNM) | I | 277 | 91.4 | |
| II | 257 | 70.1 | ||
| III | 198 | 46.5 | ||
| IV | 105 | 26.1 | ||
| Distant recurrence | Absent | 653 | 87.9 | < 0.001 |
| Visceral | 177 | 18.4 | ||
| Non-visceral | 53 | 35.9 | ||
| Locoregional recurrence | Yes | 122 | 33.5 | < 0.001 |
| No | 753 | 74.8 | ||
| Serum lactate dehydrogenase | ≤480 IU/L | 614 | 74.0 | <0.001 |
| >480 IU/L | 102 | 55.4 |
Table 8.
Gender distribution according to TNM stage
| Stage (TNM) | Male n (%) | Female n (%) |
|---|---|---|
| 0 (In Situ) | 40 (7.6) | 58 (11.1) |
| I | 122 (23.3) | 175 (33.3) |
| II | 131 (25.0) | 140 (26.7) |
| III | 122 (23.3) | 92 (17.6) |
| IV | 109 (20.8) | 59 (11.3) |
p < 0.001.
Multivariate analysis demonstrated that gender, TNM stage, LDH levels at diagnosis, the anatomic location of the primary tumour, and locoregional recurrence were associated with prognosis (Table 9) (Figure 2).
Table 9.
Multivariate analysis of melanoma-specific survival
| Variable | Category | n | HR | 95% CI (HR) | p value |
|---|---|---|---|---|---|
| Gender | Male | 188 | 1.000 | - | - |
| Female | 221 | 0.508 | 0.314; 0.822 | 0.006 | |
| Age | - | 409 | 1.005 | 0.988; 1.021 | 0.582 |
| DHL | - | 409 | 1.003 | 1.001; 1.004 | <0.001 |
| Anatomical location | Head and neck | 75 | 1.000 | - | - |
| Lower limbs | 126 | 0.575 | 0.305; 1.084 | 0.087 | |
| Upper limbs | 61 | 0.398 | 0.181; 0.878 | 0.022 | |
| Trunk | 147 | 0.429 | 0.226; 0.814 | 0.010 | |
| Histological subtype | Acral lentiginous | 30 | 1.000 | - | - |
| Nodular | 149 | 0.777 | 0.368; 1.642 | 0.509 | |
| Superficial spreading | 190 | 0.889 | 0.399; 1.981 | 0.774 | |
| Lentigo maligna | 14 | 0.516 | 0.098; 2.702 | 0.433 | |
| Not classified/other | 26 | 0.305 | 0.077; 1.207 | 0.091 | |
| Ulceration | Present | 167 | 1.000 | - | - |
| Absent | 242 | 0.902 | 0.554; 1.468 | 0.678 | |
| T | T1 | 105 | 1.000 | - | - |
| T2 | 104 | 3.005 | 1.135; 7.957 | 0.027 | |
| T3 | 84 | 2.502 | 0.862; 7.264 | 0.092 | |
| T4 | 116 | 4.264 | 1.479; 12.293 | 0.007 | |
| N | N0 | 310 | 1.000 | - | - |
| N1 | 34 | 2.143 | 1.058; 4.340 | 0.034 | |
| N2 | 31 | 2.163 | 1.072; 4.367 | 0.031 | |
| N3 | 34 | 3.249 | 1.772; 5.954 | < 0.001 | |
| M | M0 | 395 | 1.000 | - | - |
| M1 | 14 | 3.658 | 1.633; 8.192 | 0.002 | |
| Locoregional recurrence | No | 367 | 1.000 | - | - |
| Yes | 42 | 2.198 | 1.307; 3.696 | 0.003 |
Figure 2.
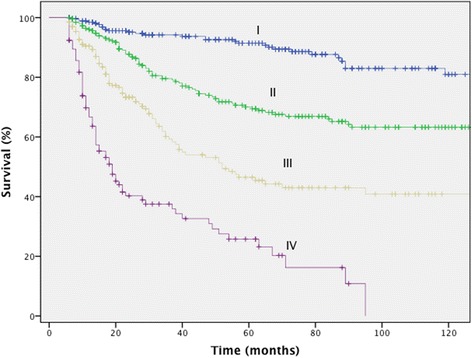
Melanoma-specific survival according to clinical stage.
Discussion
For many years, numerous studies have investigated characteristics of melanoma patients and prognostic factors. However, data from Latin America and Brazil remain scarce. The current demographic distribution of melanoma in the Brazilian population is similar to worldwide data [18], with an equal gender distribution, and a mean age of diagnosis at around the sixth decade of life. The majority of patients had white skin (91.4%). However, people in Brazil with moderately brown skin colour typically declare themselves as ‘white’, and our study did not discriminate the skin phototype [19]. Supporting the accurate self-reporting of skin colour, the acral subtype was low among the white-skinned patients (5.5%), which would likely not have been the case with mixed phototypes. The accuracy of self-reporting is also supported by the approximately one-third of non-white-skinned patients presenting with acral lentiginous melanomas.
This study included seven patients under 18 years old; two of whom died from melanoma. Although these patients may have had different predisposing conditions, they were kept in the cohort and in the survival analysis to illustrate the spectrum of the disease.
In terms of patient origin, two-thirds of patients came from Sao Paulo State and of these, only 21% lived in the region of our cancer centre. Considering that Sao Paulo State has 645 cities with total area of 248,222 km2, the majority of patients came far from their homes for treatment.
As previously found in Caucasian populations, we observed a prevalence of the superficial spreading histologic subtype, followed by nodular melanoma. The anatomic distribution of lesions showed that the trunk was a more prevalent site in men, and the lower extremities were more prevalent in women, similar to the findings of other epidemiologic studies [18].
Univariate and multivariate analyses showed higher survival rates for women. Interestingly, women more commonly presented in less advanced clinical stages, which was a factor strongly related to higher survival rates.
The used treatment modalities were in accordance with international trends. For localized melanoma, most patients underwent only surgery. The 135 (12.6%) of patients not operated on for the primary tumour are explained mainly by the 14.3% of patients presenting in clinical stage IV and patients presenting with recurrences having had previous treatment at other institutions. Sentinel node biopsy (SNB) was indicated in one-third of patients.
The overall rate of SNB was relatively low, as this procedure was only routinely performed starting in 2003. The number of pT3 and pT4 cases undergoing SNB and the mean thickness (3.2 mm) explains the high (23.4%) positivity rate. pT1 and pT2 positivity was 6.3 and 17.2%, respectively, contrasting with the 23.4% of T3 and 41.9% of T4 tumours, showing the correlation of positivity with thickness. In the literature, the positivity rate varies from 12 to 26% [20-23]. The 9.8% rate of locoregional recurrence among cases with negative SNB is high. However, this rate includes not only nodal lesions but also local recurrence and in-transit metastases. There were a significant number of patients (262, 24.4%), with no Breslow information. Of these, 145 (54%) had stage III and IV disease. SNB was performed in this situation in 54 cases (14.5%). Unfortunately, our centre receives many patients with previous excisional biopsy and no Breslow thickness or other histopathological information. We tried to reanalyse the slides and paraffin blocks, but this was not possible for some cases. In such situations we lowered the threshold for performing SNB for localized disease (clinical stage I and II).
Locoregional recurrence is not usually emphasized, but was strongly related to prognosis, with significantly lower survival rates, which indirectly shows the importance of correct surgical treatment.
Systemic therapy at our institution was chemotherapy and interferon alpha, which was indicated for systemic disease (Stage IV or recurrence) or inoperable Stage III. Surgery for metastatic disease was indicated frequently, mainly for non-visceral disease. First-line chemotherapy was dacarbazine. Second-line was carboplatin and paclitaxel or immunotherapy with interferon alpha. Target therapy or modern immunotherapies are currently in use at the institution, but were not applied to this population.
Radiation therapy was indicated mostly to palliate metastases, and in selected cases, as an adjuvant setting after lymphadenectomy. Indications were bulky tumours (general) and head and neck lymph node metastasis. This course of treatment is not well established but there is evidence for its use in selected cases [24].
The overall 5-year survival rate of 67.6% for all patients was low, but when stratified by clinical stage, it was very similar to other larger series, including the AJCC. These findings show that the higher number of advanced stage cases impacted the overall survival. This is somewhat expected in data from tertiary cancer centres, but is the opposite of findings from developed countries with successful secondary skin cancer prevention policies—related to early diagnosis—which have achieved an overall survival of over 90% [10,25,26].
The presence of intratumoural lymphocyte infiltration, a known factor related to prognosis, did not affect survival rates in this study. Vascular and/or lymphatic infiltration were related to a lower survival rate, but not to a statistically significant degree. Other factors, including mitotic index, sentinel node status, and microscopic satellitosis, associated with prognosis in the univariate analysis, could not be tested in the multivariate model, because a relatively small number of patients remained in the model. The lack of information from referring sites impaired more inclusive analysis. A large number of patients had also had their tumours excised in other centres with little or no histological information on the primary tumour. However, most cases were suitable for univariate analysis and at least 400 cases were pooled in the multivariate analysis.
The overall survival for the entire cohort was lower than that observed in Caucasian melanoma populations. As expected, our data confirmed that the major factors related to melanoma prognosis are TNM stage, LDH levels, and locoregional recurrence. Patients with head and neck tumours had lower survival rates than patients with trunk and upper extremity disease. Anatomic location, besides not being completely accepted as a prognostic factor, has been associated with prognosis in other studies, and was independently related to survival rate in our cohort [15]. Other well-known prognostic factors, such as ulceration and mitotic index, were found to be associated with survival only in the univariate analysis. This may be related to the relatively small number of patients in the present study, compared with the more than 17,000 cases that validate the UICC/AJCC staging system [8]. Other factors related to prognosis only in the univariate analysis that were not confirmed as prognosticators included age, acral lentiginous subtype, Clark level, and microscopic satellitosis.
Conclusions
This study shows that Brazilian melanoma patients experienced a lower survival rate than the current worldwide average. Our findings confirm the strong association between TNM stage and local recurrence to prognosis. The high prevalence of advanced cases reinforces the importance of local strategies to diagnose melanomas in the early stages, and to treat it definitively. This retrospective study from a single institution has several limitations, such as incomplete data and selection bias and it remains important to perform multi-institutional prospective studies to attain a better understanding of possible socioeconomic and racial disparities in Brazil and Latin America that may affect access to treatment.
Methods
The study population included all patients diagnosed with melanoma between January 1997 and December 2011 at the Barretos Cancer Hospital (BCH) in Barretos, Sao Paulo State, Brazil. The BCH is a non-profit institution focused on public health and is a high-volume tertiary cancer centre, treating patients from all over the country free of charge [27]. All data were collected from medical records following the appropriate ethical guidelines, and the local ethics committee approved the study (Barretos Cancer Hospital Ethics Committee, #548/2011).
Data analyses were performed with software (SPSS for Windows®, SPSS Inc., Chicago IL, USA). Patients who were previously treated at other institutions were included as long as their records included sufficient information and at least 6 months of follow-up at the BCH. Patients seen for second opinions with incomplete treatment were excluded, as well as all in situ lesions. For survival analysis, at least 24 weeks of follow-up were required. The variables were categorized as demographics (gender, patient origin, age, and skin colour), clinical/histological data (anatomic location of primary tumour; level of serum LDH at first consultation; histological subtype; Breslow depth; Clark level; presence of ulceration; mitotic index; perineural, vascular, and lymphatic infiltration; and peri- and intratumoural lymphocyte infiltration), and treatment-related factors (surgery of primary tumour, sentinel node biopsy, systemic therapy, and radiation therapy).
Indications for sentinel node biopsy were tumour thickness ≥ 0.75 mm. Indications for SNB in tumours between 0.75 mm and 1.00 mm thickness were the presence of mitosis or ulceration. Stage IV and clinically diagnosed stage III were contraindications to SNB.
Skin colour was recorded by the clinician. When this information was missing, it was collected from patient identification, which is registered based on self-report. Clinical stage was determined using the 2009 UICC TNM system [10]. Stages were not stratified into subdivisions for analysis. DSS analyses were performed according to the Kaplan–Meier method, using the log-rank test to compare survival curves. For the continuous variable age, we used the Cox proportional hazards regression method; the variables associated with prognosis (p < 0.05 in univariate analysis) were included in the Cox regression multivariate model analysis if at least 400 cases were available for testing, with the calculation of the hazard ratio (HR) for death and modelling according to variables of interest. Age, TNM stage, and ulceration were used as adjusted model variables, regardless of statistical significance [28]. Statistical significance was set as 5% for all tests.
Acknowledgements
This work was supported by grant # 2012/4194-1, from the São Paulo Research Foundation (FAPESP).
Abbreviations
- AJCC
American Joint Committee on Cancer
- BCH
Barretos Cancer Hospital
- DSS
Disease-specific survival
- HR
Hazard ratio
- LDH
Lactate dehydrogenase
- SD
Standard deviation
- SNB
Sentinel node biopsy
- UICC
International Union Against Cancer
- UV
Ultraviolet
Footnotes
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
VLV was involved in the study conception, data analysis, and writing of the paper. TBS, MAV, ATTO, MVL, and DAPA collected the data, and reviewed the results and the final manuscript. JHTGF and ECC were involved in the data analysis, and reviewed the results and final manuscript. All authors read and approved the final document.
Contributor Information
Vinicius de Lima Vazquez, Email: viniciusvazquez@gmail.com.
Thiago Buosi Silva, Email: tbuosi@gmail.com.
Marcelo de Andrade Vieira, Email: mvieiraonco@gmail.com.
Antônio Talvane Torres de Oliveira, Email: netto123@uol.com.br.
Marcílio Vital Lisboa, Email: vitalisboa@gmail.com.
Diocésio Alves Pinto de Andrade, Email: diocesio@yahoo.com.
José Humberto Tavares Guerreiro Fregnani, Email: mdfregnani@terra.com.br.
Estela Cristina Carneseca, Email: estela.federal@gmail.com.
References
- 1.Uong A, Zon LI. Melanocytes in development and cancer. J Cellular Physiol. 2010;222(1):38–41. doi: 10.1002/jcp.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr.
- 3.Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17–S25. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 4.Brasi. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Coordenação Geral de Ações Estratégicas. Coordenação de Prevenção e Vigilância: Estimativa 2014: Incidência de Câncer no Brasil. INCA, Rio de Janeiro, 2013 [in Portuguese]
- 5.Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Coordenação Geral de Ações Estratégicas. Coordenação de Prevenção e Vigilância: Atlas de Mortalidade por Câncer. INCA, Rio de Janeiro, 2014 [in Portuguese].
- 6.Fears TR, Guerry D, Pfeiffer RM, Sagebiel RW, Elder DE, Halpern A, et al. Identifying individuals at high risk of melanoma: a practical predictor of absolute risk. J Clin Oncol. 2006;24(22):3590–3596. doi: 10.1200/JCO.2005.04.1277. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Soong S, Ross MI, Urist MM, Karakousis CP, Temple WJ, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7(2):87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 9.Roulin D, Matter M, Bady P, Lienard D, Gugerli O, Boubaker A, et al. Prognostic value of sentinel node biopsy in 327 prospective melanoma patients from a single institution. Eur J Surg Oncol. 2008;34(6):673–679. doi: 10.1016/j.ejso.2007.07.197. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18(22):3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 12.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Ploeg AP, van Akkooi AC, Haydu LE, Scolyer RA, Murali R, Verhoef C, et al. The prognostic significance of sentinel node tumour burden in melanoma patients: an international, multicenter study of 1539 sentinel node-positive melanoma patients. Eur J Cancer. 2014;50(1):111–120. doi: 10.1016/j.ejca.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Spillane AJ, Pasquali S, Haydu LE, Thompson JF. Patterns of recurrence and survival after lymphadenectomy in melanoma patients: clarifying the effects of timing of surgery and lymph node tumor burden. Ann Surg Oncol. 2014;21(1):292–299. doi: 10.1245/s10434-013-3253-6. [DOI] [PubMed] [Google Scholar]
- 15.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Ding S, Byrd DR, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmerling RA, Loria D, Cinat G, Ramos WE, Cardona AF, Sanchez JL, et al. Cutaneous melanoma in Latin America: the need for more data. Revista Panam Salud Publica. 2011;30(5):431–438. doi: 10.1590/S1020-49892011001100005. [DOI] [PubMed] [Google Scholar]
- 17.Bagaria SP, Faries MB, Morton DL. Sentinel node biopsy in melanoma: technical considerations of the procedure as performed at the John Wayne Cancer Institute. J Surg Oncol. 2010;101(8):669–676. doi: 10.1002/jso.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther. 2010;10(11):1811–1823. doi: 10.1586/era.10.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzpatrick TB. Ultraviolet-induced pigmentary changes: benefits and hazards. Curr Probl Dermatol. 1986;15:25–38. doi: 10.1159/000412090. [DOI] [PubMed] [Google Scholar]
- 20.Kruper LL, Spitz FR, Czerniecki BJ, Fraker DL, Blackwood-Chirchir A, Ming ME, et al. Predicting sentinel node status in AJCC stage I/II primary cutaneous melanoma. Cancer. 2006;107(10):2436–2445. doi: 10.1002/cncr.22295. [DOI] [PubMed] [Google Scholar]
- 21.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 22.Paek SC, Griffith KA, Johnson TM, Sondak VK, Wong SL, Chang AE, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer. 2007;109(1):100–108. doi: 10.1002/cncr.22382. [DOI] [PubMed] [Google Scholar]
- 23.Woods JFC, De Marchi JA, Lowery AJ, Hill ADK: Validation of a nomogram predicting sentinel lymph node status in melanoma in an Irish population. Ir J Med Sci (1971-) 2014:1-5 [DOI] [PubMed]
- 24.Burmeister BH, Henderson MA, Ainslie J, Fisher R, Di Iulio J, Smithers BM, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589–597. doi: 10.1016/S1470-2045(12)70138-9. [DOI] [PubMed] [Google Scholar]
- 25.MacKie RM, Bray C, Vestey J, Doherty V, Evans A, Thomson D, et al. Melanoma incidence and mortality in Scotland 1979–2003. Br J Cancer. 2007;96(11):1772–1777. doi: 10.1038/sj.bjc.6603801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK (eds). SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda, MD: http://seer.cancer.gov/csr/1975_2007/
- 27.Carneseca EC, Mauad EC, de Araujo MA, Dalbo RM, Longatto Filho A, Vazquez Vde L. The Hospital de Cancer de Barretos Registry: an analysis of cancer survival at a single institution in Brazil over a 10-year period. BMC Res Notes. 2013;6:141. doi: 10.1186/1756-0500-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox DR. Regression Models and Life-Tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]


