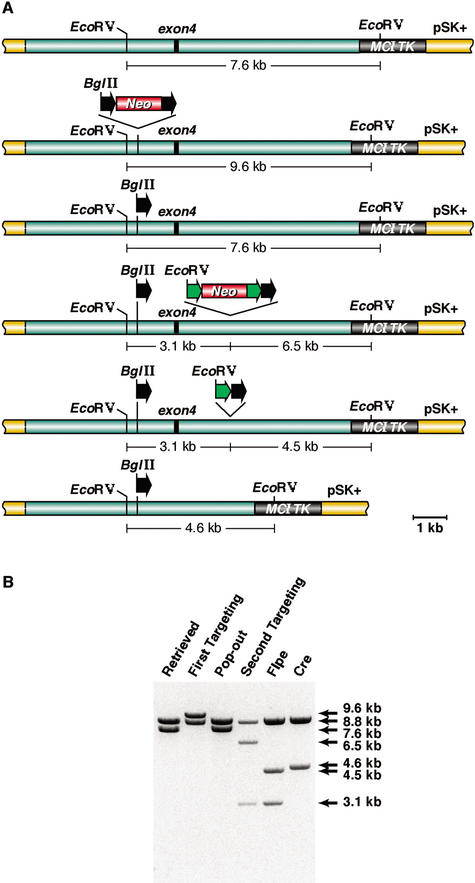
Figure 3.
Constructing an Evi9 conditional knockout allele. (A) The 11.0-kb genomic DNA fragment containing Evi9 exon 4 was subcloned from BAC-A12 using gap repair. EcoRV digestion of the gap-repaired plasmid generates 7.6-kb and 8.8-kb fragments. The 7.6-kb fragment contains Evi9 exon 4 sequences, whereas the 8.8-kb fragment, common to all lanes, contains plasmid sequences and Evi9 sequences located upstream of exon 4. The floxedNeo cassette of PL452 was targeted upstream of Evi9 exon 4. In the targeted plasmid, the 7.6-kb EcoRV fragment increases in size to 9.6 kb because of the addition of the floxedNeo cassette. Excision of the floxed Neo cassette leaves behind a single loxP (black arrow) at the targeted locus, and the normal EcoRV digestion pattern is restored. Next, the PL451 selection cassette, containing the Neo gene flanked by FRT sites (green arrow) and a downstream loxP, was targeted downstream of Evi9 exon 4. The PL451 selection cassette contains an EcoRV site, which results in the production of 6.5-kb and 3.1-kb fragments following EcoRV digestion. This is the Evi9 cko-targeting vector. To test the functionality of the FRT sites in the cko-targeting vector, the PL451 selection cassette was excised from the cko-targeting vector by FLP recombinase following electroporation into EL250 cells. This reduces the size of the 6.5-kb EcoRV fragment to 4.5 kb. Finally, electroporation of the cko-targeting cassette into EL350 cells expressing Cre recombinase excises the entire DNA between the two loxP sites, creating a 4.6-kb EcoRV fragment. (B) EcoRV-digestion patterns of the plasmids at every stage of the targeting vector construction.